Redox reaction
advertisement

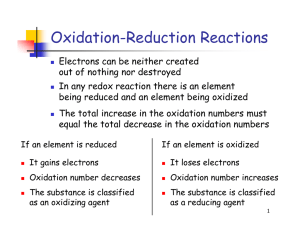
Definition If oxidation occurs, then reduction must also occur Any chemical process in which elements undergo a change in oxidation number is a Redox reaction Example 2Na + Cl2 → 2Na+ + 2ClThis reaction can be thought of as two half reactions 2 Na → 2Na+ + 2e Cl2 + 2e → 2Cl- oxidation reduction These two reactions together are called a redox reaction Identifying Redox SO2 + H2O + O2 → H2SO4 NaCl + Ag(NO3) → Na(NO3) + AgCl Practice Determine if each below is a redox reaction SO3 + H2O → H2SO4 2KNO3 → 2KNO2 + O2 H2 + CuO → Cu + H2O More Definitions Reducing Agents – elements that undergo oxidation (the cause reduction in another element) Oxidizing Agents – undergo reduction Mg + Cu(NO3) → Cu + Mg(NO3) Mg is reducing agent – being oxidized Cu2+ is oxidizing agent – being reduced Corrosion When a metal is reduced When two metals are in contact, the one that is the better reducing agent (most active) will give up electrons to the other one The metal that receives the electrons is reduced – called corrosion When zinc is wrapped around the Fe nail, Zn is oxidized, so Fe corrodes When copper is wrapped around the Fe nail, Fe is oxidized, so Cu corrodes