Oxidation-Reduction Reactions: Balancing Redox Equations
advertisement
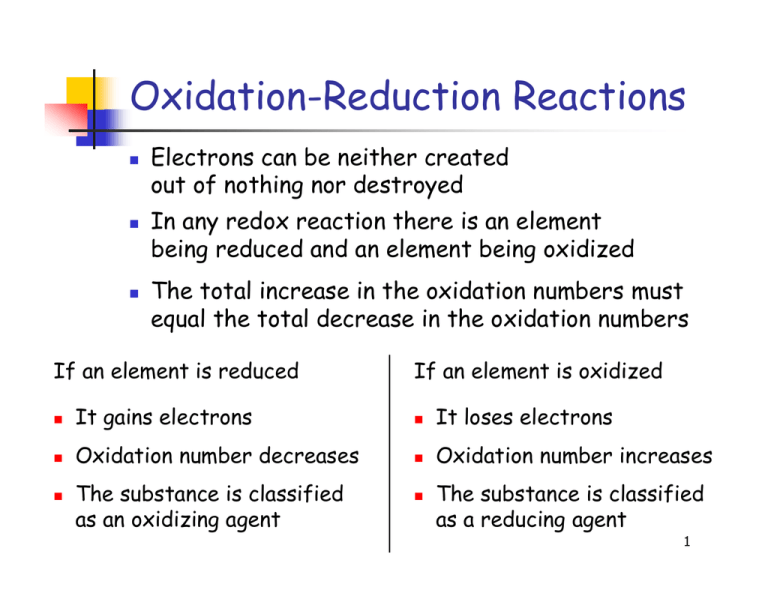
Oxidation-Reduction Reactions Electrons can be neither created out of nothing nor destroyed In any redox reaction there is an element being reduced and an element being oxidized The total increase in the oxidation numbers must equal the total decrease in the oxidation numbers If an element is reduced If an element is oxidized It gains electrons It loses electrons Oxidation number decreases Oxidation number increases The substance is classified as an oxidizing agent The substance is classified as a reducing agent 1 Balancing Redox Reactions 1. Determine oxidation numbers for all elements in each compound involved in the reaction 2. Separate the oxidation and reduction processes and write them as half-reactions 3. Balance each half-reaction by inspection and add the necessary number of electrons to balance the charge 4. Multiply the half-reactions by integer numbers to equalize the numbers of electrons gained and lost in each 5. Add the half-reactions and cancel any common terms to get the balanced equation 2 Example 1 Balance the following redox reaction. Determine the oxidizing and reducing agents and write the net ionic equation. KMnO4 + KCl + H2SO4 → MnSO4 + K2SO4 + H2O + Cl2 3 Example 2 Balance the following redox reaction. Determine the oxidizing and reducing agents and write the net ionic equation. HNO3 + H2S → NO + S + H2O 4 Example 3 Balance the following redox reaction. Determine the oxidizing and reducing agents and write the net ionic equation. Zn + NaNO3 + NaOH + H2O → Na2[Zn(OH)4] + NH3 5 Example 4 Balance the following redox reaction. Determine the oxidizing and reducing agents and write the net ionic equation. NaHSO4 + Al + NaOH + H2O → Na2S + Na[Al(OH)4] 6 Example 5 Balance the following redox reaction. Determine the oxidizing and reducing agents and write the net ionic equation. CoCl2 + Na2O2 + NaOH + H2O → Co(OH)3 + NaCl 7 Example 6 Balance the following redox reaction. Determine the oxidizing and reducing agents and write the net ionic equation. K2Cr2O7 + HCl → CrCl3 + Cl2 + H2O + KCl 8 Example 7 The citrate ion, C2O4–, is oxidized by the permanganate ion, MnO4–, in the sulfuric acid solution, forming carbon dioxide and Mn2+ ion. Write and balance the net ionic equation, and then derive the formula unit equation for this reaction. 9 Example 8 525 mL of iodine solution was titrated with 7.28 mL of 0.2 M nitric acid solution producing iodic acid and nitrogen(IV) oxide. What is the concentration of the iodine solution? 10 Example 9 What mass of N2H4 can be oxidized to N2 by 24.0 g K2CrO4, which is reduced to Cr(OH)4– in basic solution? 11 Assignments & Reminders Read Chapter 11 completely Read Section 4-7 of Chapter 4 Review Session – 5:30 to 7:00 pm TODAY in 107 Heldenfels Review Session – 5:00 to 7:00 pm TOMORROW in 105 Heldenfels 12







