Science 1206 - Unit2 : Chemistry Test Review: Topic 1: WHMIS
advertisement

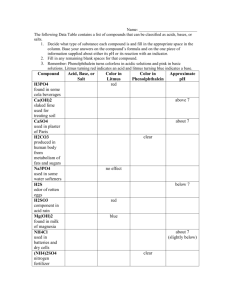
Science 1206 - Unit2 : Chemistry Test Review: Topic 1: WHMIS / MSDS WHMIS: Workplace Hazardous Materials Information System. There are 8 WHMIS symbols, you are required to recognize the 8 symbols and the hazards that they identify. MSDS: Material Safety Data Sheet. Define what a MSDS is, why it is important, and know the 9 required sections on an MSDS. Topic 2: Evidence of physical change. Be able to give examples of evidence that chemical change has occurred. (Reference your foldable) Examples: A new colour appears, Heat or light given off, gas bubbles form, precipitate forms, process is difficult to reverse. Topic 3: The periodic table. Know the 4 main sections of the periodic table (metals, non-metals, metalloids, noble gases). If given an element, be able to classify it into one of the 4 sections. Topic 4: Naming Ionic Compounds Given the name of a compound, determine the formula. (Using the criss-cross method may be useful) Examples: Barium Fluoride Ba2+ F- BaF2 Iron(III) Oxide Fe3+ O2- Fe(III)2O3 Calcium Sulfide Ca2+ S2- Ca2S2 (can be written in a lower ratio) CaS Given the formula of a compound, determine the name. Examples: Zn3P2 Zinc Phosphide Cu(II)O Copper(II) Oxide AuBr Gold Bromide CsCl Cesium Chloride Given the name of an ionic compound with a polyatomic ion, determine the formula. Examples: Magnesium Hydroxide Mg2+ OH- Mg(OH)2 Ammonium Chloride NH4+ Cl- NH4Cl Chromium(III) Dichromate Cr3+ Cr2O72- Cr2(Cr2O7)3 Given the formula of a polyatomic ionic compound, determine the name Examples: AgIO3 Silver Iodate NH4CN Ammonium Cyanide Os(HPO4)2 Osmium Hydrogen Phospate Sr(C6H5COO)2 Strontium Benzoate Topic 5: Naming Molecular Compounds Given the name of the molecular compound, determine the molecular formula Examples: Dinitrogen Tetrahydride N2H4 Carbon Dioxide CO2 Dichlorine Monoxide Cl2O Nitrogen Tribromide NBr3 Given the formula of the molecular compound, determine the name. Examples: S2H2 Disulfur Dihydride SiO2 Silicon Dioxide SiC Silicon Monocarbide C6H8 Hexacarbon Octahydride 1 = mono , 2 = di , 3 = tri , 4 = tetra , 5 = penta , 6 = hexa , 7 = hepta , 8 = octa , 9 = nona , 10 = deca Topic 6 : Hydrated Ionic Compounds Hydrated compounds contain water. Given the name of the compound determine the formula, or if given the formula determine the name. Examples: CuSO4∙5H2O Copper Sulfate Pentahydrate Cobalt(II) Chloride Dihydrate Co(II)Cl2 ∙2H2O Topic 7 : Energy levels and valence shells Electrons are distributed in fixed patterns around the nucleus. The nucleus contains protons (+) and neutrons (neutral). The number of protons in an atom cannot change, and can be determined by the atomic number (i.e. Nitrogen has an atomic number of 7, so it has 7 protons). A neutral atom has equal numbers of protons and electrons. (total charge = #protons #electrons) Electrons fill in energy levels by occupying the lowest energy levels first, then the higher energy levels. ( 2e- in the first shell, 8e- in the next, 8e- in the third, 18e- in the fourth…) The outer energy level is known as the valence shell. Atoms are stable when the outer shell is filled. This is why atoms tend to lose or gain electrons to become ions. Other atoms will share electrons to fill their outer shell. This is known as covalent bonding. Example: Sulfur Valence Shell : 6 eThis is the energy diagram for sulfur. We know it is sulfur because it has 16 protons, and 16 is the atomic number for sulfur. A neutral sulfur atom will have 16 electrons as well. (2 ein the first shell, 8 e- in the second shell and the rest will be in the valence shell , 6e-) Valence Shell : 8eSulfur will try to fill its outer shell to become more stable. It can do that by gaining 2 extra electrons. It will then have 18e- and 16 protons. The charge will be 16 -18 = -2. Topic 8 : Properties of Ionic and Molecular Compounds Know the general properties of ionic and molecular compounds: Ionic Compounds - Solids at room temperature, May or may not be soluble in water, may form a coloured solution in water if soluble or as a liquid, conduct electricity if soluble, melting points usually above 300OC. Molecular Compounds – May be solids, liquids or gases at room temperature, may or may not be soluble in water, generally form colourless solutions in water, do not conduct electricity if soluble, melting points usually below 300OC Topic 9: Molecular and Empirical Formulas, molecule, molecular element An empirical formula is the lowest ratio of one atom to another in a compound. A molecular formula is the exact number of each atom in a compound A molecule is when two or more atoms bond together A molecular element is when two or more of the same element bond together o 7 elements exist as Dimolecular elements in nature o Hydrogen, Nitrogen, Oxygen, Fluorine, Chlorine, Bromine, Iodine Examples: Given the molecular formula, determine the empirical formula: C2H6 CH3 P4Cl10 P2Cl5 NOTE: If you are given the empirical formula, you CANNOT determine the molecular formula. Topic 10 : Acids and Bases Acids: Acids are Molecules that ionize in water to produce H+ ions, which give acids their properties. Properties of Acids: Conduct Electricity Turn blue litmus paper red Taste Sour React with many metals to produce hydrogen gas, H2(g) Have a pH value less than 7 Neutralize or partially neutralize bases Naming acids: General Rules Name the hydrogen compound like an ionic compound. This is the compound name before it is placed in water. Then determine the name of the acid when the compound is placed in water. 1. Hydrogen ______ ide becomes hydro_____ ic acid eg. HCl Hydrogen Chloride Hydrochloric Acid 2. Hydrogen ite becomes ous acid Eg. HClO Hydrogen Chlorite Chlorous Acid 3. Hydrogen ___ate becomes ________ic acid Eg. H2SO4 Hydrogen Nitrate Nitric Acid Carboxylic Acids: Carboxylic Acids are compounds that end in COOH. The Hydrogen in COOH is the acidic hydrogen, and These acids are commonly referred to as organic acids. Example: CH3COOH – Acetic Acid (Vinegar) Bases: Ionic compounds that contain the hydroxide ion OH-, an ion that gives a base it’s properties. Properties of Bases: Conduct electricity Turn Red Litmus Paper Blue Taste Bitter Feel Slippery Have a pH value greater than 7 Neutralize or partially neutralize acids Naming Bases: Bases are named exactly as the ionic compound is named Eg. NaOH Sodium Hydroxide Eg. Ca(OH)2 Calcium Hydroxide Writing the formula for a base: Follow the rules for ionic compounds Eg. Barium Hydroxide Ba2+ OH- Criss Cross Method Ba(OH)2 Terms to Know: Chemistry , Matter , Ionic Compound , Molecular Compound , Valence Shell , Proton , Neutron , Electron , WHMIS , MSDS , soluble, insoluble, electrical conductivity, melting point, chemical property, physical property, solid , liquid , gas.