Percent Composition Worksheet: Chemistry Practice Problems
advertisement
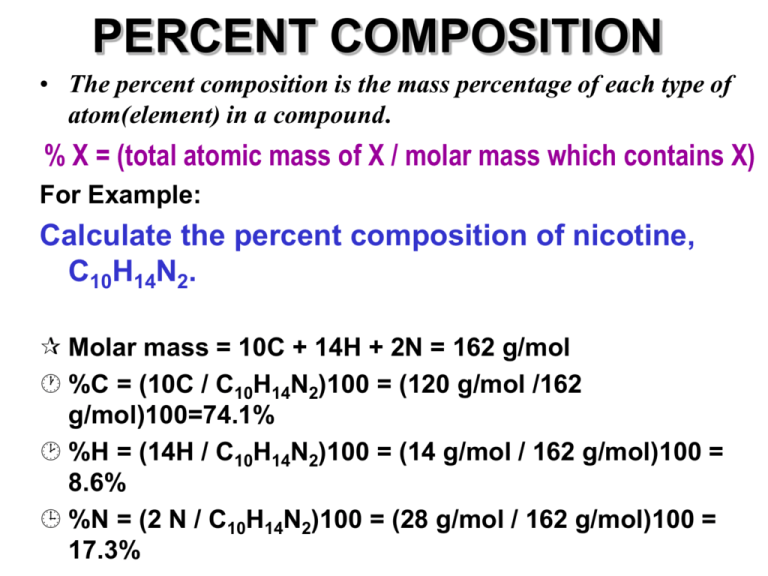
PERCENT COMPOSITION • The percent composition is the mass percentage of each type of atom(element) in a compound. % X = (total atomic mass of X / molar mass which contains X) For Example: Calculate the percent composition of nicotine, C10H14N2. Molar mass = 10C + 14H + 2N = 162 g/mol %C = (10C / C10H14N2)100 = (120 g/mol /162 g/mol)100=74.1% %H = (14H / C10H14N2)100 = (14 g/mol / 162 g/mol)100 = 8.6% %N = (2 N / C10H14N2)100 = (28 g/mol / 162 g/mol)100 = 17.3% PERCENT COMPOSITION • The percent composition can also be calculated from experimental data. % X = (total mass of X / total mass of compound) A student prepares a compound of tungsten chloride from 3.946 g of tungsten and 3.806 g of chlorine. Assuming the reaction goes to completion, calculate the percent composition. According to the Law of Conservation of Mass, 3.946 g of W combines with 3.806 g of Cl to give 7.752 g of compound. %W = (mass W / mass WxCly )100 = (3.946 g / 7.752 g)100 = 50.9% %Cl = (Cl / WxCly )100 = (3.806g / 7.752 g)100 = 49.1% PERCENT COMPOSITION WORD PROBLEMS Q. How many grams of lithium will combine with 20.0 g of sulfur to form Li2S? Molar mass of Li2S = 2Li + S = 2(6.94 g/mol) + 32.06 g/mol =45.94 g/mol % composition of Li in Li2S = (mass of Li/molar mass)100 = (13.88 g/mol / 45.94 g/mol) 100 = 30.2% % composition of S in Li2S = 100% - 30.2% =69.8% A ratio can now be established: 20.0 g S = 69.8% x = (30.2%) (20.0 g) x = 8.65 g x g Li 30.2% 69.8% PRACTICE PROBLEMS #11 A _____1. A 45.9 g sample of Copper, Cu, combined with oxygen to give 51.69 g of an oxide compound. The percent composition of this compound is: a) 88.8% Ca, 11.2% O b) 24.4% Ca, 75.6% O c) 60.1% Ca, 39.9% O d) 11.3% Ca, 88.7% O 2. Calculate the percent composition for each set? 11.1% 88.9% H2O H =_____ O = _____ H2O2 5.88% H =_____ 94.1% O = _____ 3. How many grams of sodium will combine with 567.0 g of sulfur to form Na2S?. 815.6 g GROUP STUDY PROBLEMS #11 ______1. A 5.016 g sample of chromium, Cr, combined with oxygen to give 7.333 g of an oxide compound. The percent composition of this compound is: a) 59.1% Cr, 40.9% O b) 24.4% Cr, 75.6% O c) 68.4% Cr, 31.6% O d) 71.5% Cr, 28.5% O 2. Calculate the percent composition. K2CrO4 K =_____ Cr =_____ O = _____ K2Cr2O7 O = _____ K =_____ Cr =_____ 3. How many grams of aluminum will combine with 3.67 g of sulfur to form Al2S3?







