Ppt05(Wk11)_ch18 - Oakton Community College
advertisement

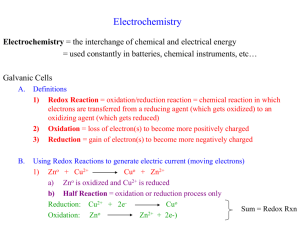
Electrochemistry • “Marriage” of redox and thermo • Spontaneous electron-transfer reactions can result in spontaneous electric current if the reactants are separated by a wire – Voltaic (Galvanic) cells [Experiment 32!] – We can use the “spontaneity” of the reaction to do electrical work 1 (Continued) • We can “push” electrons through a cell in order to make a nonspontaneous redox reaction occur. – Electrolysis cell [Experiment 32!] – Doing work to “force” chemical reaction to occur [opposite of voltaic cell] 2 Balancing Redox Equations • Deferred until later • For now just know that: – A half reaction has electrons written as a reactant or a product • Oxidation half reaction: A reactant gets oxidized (loses electrons); electrons appear as a “product” • Reduction half reaction: A reactant gets reduced (gains electrons); electrons appear as a “reactant” – A balanced redox equation does not show electrons explicitly. #e-’s lost = #e-’s gained (called “n”) 3 Voltaic Cells • Recall lab… 4 5 • The spontaneous rxn occurs in the cell • e-’s flow from – to + (“get to go where they want to go”) • Anode = where ox occurs • Cathode = where red occurs • Salt bridge prevents charge buildup (which would stop flow) 6 7 Used when neither redox species in a half reaction (or electrode) is a neutral metal. You used graphite in place of Pt in lab for Fe2+/Fe3+ and I2/Icells. A cheaper “inert” electrode. Neither is a neutral metal 8 Standard Reduction Potentials (E°red) • Recall lab – Make a bunch of different cells, get different Ecell values (Eºcell if at standard state). – Clearly some reductions are more favorable than others • How do you know? [Which direction did e-’s flow?] • By how much? • Rank them? (Must pick a zero as reference.) 9 Quick quiz • NOTE: Every electrode compartment has one oxidizing agent and one reducing agent (this pair is called the redox “couple”) • If an electrode has Ni(s) and Ni2+ ions in it, which species is the oxidizing agent and which the reducing agent (of the pair)? Ni2+ (b/c it’s “more positive”; it has Ox agent is ___ “room for an electron” Ni (b/c it’s “more negative”; it Red agent is ___ has an electron to give) 10 Revisit Earlier Cell—Look at this as a “Competition for the electrons”. Which oxidizing agent “wants them more”? Who is the (possible) oxidizing agent on Zn2+ the left? _____ Hint: The two “players” are Zn and Zn2+ Who wins? (Which one “got” the Cu2+ electrons?) ____ Who is the (possible) oxidizing agent on Cu2+ the right? _____ Hint: The two “players” are Cu and Cu2+. Cu2+ “pulled harder” So…which of the half reactions shown at the right is more favorable (greater tendency to happen)? Cu2+ + 2 e- Cu(s) Zn2+ + 2 e- Zn(s) By how much?..... 11 Reducing Cu2+ is more favorable than reducing Zn2+ …by 1.10 V! (Measure it w/voltmeter!) We define a “standard reduction potential”, E°red, for every reduction half reaction such that: E°cell = E°red(cathode) - E°red(anode) Where reduction takes place The more positive the “E” (Ecell, Ered, or Eox), the more favorable the process 12 Reducing Cu2+ is more favorable than reducing Zn2+ …by 1.10 V! (Measure it w/voltmeter!) E°cell = E°red(cathode) - E°red(anode) 1.10 V = E°red(Cu2+/Cu) - E°red(Zn2+/Zn) NOTE: If E°red(Zn2+/Zn) were 0 V, E°red(Cu2+/Cu) would be +1.10 V If E°red(Zn2+/Zn) were -1.0 V, E°red(Cu2+/Cu) would be +0.10 V If E°red(Zn2+/Zn) were +1.0 V, E°red(Cu2+/Cu) would be +2.10 V 13 The “zero” is arbitrary, but must be chosen / agreed upon! This electrode (SHE) was ultimately the one chosen by the scientific community to be the “zero” of potential. 2 H+ + 2 e- H2 (g); E°red = 0.0 V Upshot: One can determine any E°red experimentally by just setting up a cell where one of the half cells is SHE! (next slide → ) 14 Determining a Standard Reduction Potential using the SHE 0.76 V = E°red(SHE) - E°red(Zn2+/Zn) 0.76 V = 0 - E°red(Zn2+/Zn) E°red(Zn2+/Zn) = - 0.76 V The reduction of H+ is more favorable than the reduction of Zn2+…. by 0.76 V! Ecell = Ecathode - Eanode Both as reductions 15 H+ (not Zn2+) gets reduced Could use this info to predict that this direct reaction would occur: Use these values to: predict which reactions are spontaneous at standard state and to find any E°cell! E°cell = E°red(cat) - E°red(an) 2 H+(aq) + 2 e- H2(g) 0 E°cell = 0 – (-0.76) = +0.76 V Zn2+(aq) + 2 e- Zn(s) -0.76 H+ gets reduced; Zn2+ does not (Zn gets oxidized): 2 H+ + Zn → H2(g) + Zn2+ is spontaneous: E°cell > 0 16 Refers only to species on the left side of the arrow. E.g., F2, is a better ox agent than H2O2 which is better ox agent than Au3+ (but all of these species are very good oxidizing agents relative to most!) Refers only to species on the right side of the arrow. E.g., F-, is a poorer red agent than H2O which is poorer red agent than Au(s) (but all of these are very poor reducing agents relative to most!) 17 Excerpt from Voltaic Cell lab reading 18 Table 18.1 (continued) 19 Recall earlier slide We define a “standard reduction potential”, E°red, for every reduction half reaction such that: E°cell = E°red(cathode) - E°red(anode) OR (could also write Ecell as… Ecell = Ered + Eox 20 Lab interlude • See overhead / board • The lab manual initially asks you to pretend that the Ag+/Ag reduction potential is 0.0 V just to show you the “arbitraryness” of this. • Then it tells you that in reality, Ag+/Ag reduction potential is 0.80 V if the H+/H2 potential (SHE) is 0.0 V 21 Cu/Cu2+ & Fe3+/Fe2+ 0.32 V Cu/Cu2+ Cu Cu2+ + 2e- Zn/Zn2+ & Ag/Ag+ 1.50 V Zn/Zn2+ Zn Zn2+ + 2e- 1.50 V Ag+ + e- Ag 0.0 V Zn/Zn2+ Zn Zn2+ + 2e- 1.50 V Cu2+ + 2e- Cu -0.45 V Cu/Cu2+ & Zn/Zn2+ 1.05 V +0.45 V Fe3+ + e- Fe2+ -0.13 V **Circle the species that is the better oxidizing agent** 22 From Text Table From Table A.1: Fe3+ + e- Fe2+ -0.13 V Ag+ + e- Ag 0.0 V -0.13 V -0.45 V 0.0 V -1.50 V (See next slide) Ag+ + e- Ag 0.80 V 0.80 V Fe3+ + e- Fe2+ 0.67 V 0.77 V Cu2+ + 2e- Cu 0.35 V 0.34 V Zn2+ + 2e- Zn -0.70 V -0.76 V Cu2+ + 2e- Cu -0.45 V Zn Zn2+ + 2e- 1.50 V Zn2+ + 2e- Zn -1.50 V Flip 23 24 Table 18.1 (continued) 25 Determine the cell reaction, calculate Ecell , identify the cathode and anode, label the (+) and (-) electrodes, and show electron flow (see board) Ni2+(aq) + 2 e- Ni(s) -0.23 V Mn2+(aq) + 2 e- Mn(s) -1.18 V The better oxidizing its Ered is Ni2+ (Because agent is:___ more positive) Ni2+ actually gets So ___ reduced, and thus electrons flow to the right __ side, which _____ must be the ____ode. cath So the Ni electrode must be ______ive posit V V - -1.18 E°cell = -0.23 _____ _____ +0.95 V = _______ 26 Determine the cell reaction, calculate Ecell , identify the cathode and anode, label the (+) and (-) electrodes, and show electron flow (see board) Fe2+(aq) + 2 e- Fe(s) -0.45 V Mg2+(aq) + 2 e- Mg(s) -2.37 V The better oxidizing Fe2+ agent is:___ Fe2+actually gets So ___ reduced, and thus electrons flow to the right __ side, which _____ must be the ____ode. cath So the Fe electrode must be ______ive posit V V - -2.37 E°cell = -0.45 _____ _____ +1.92 V = _______ 27 Determine the cell reaction, calculate Ecell , identify the cathode and anode, label the (+) and (-) electrodes, and show electron flow (see board) e Pb(s) Fe2+(aq) + 2 e- Fe(s) Pb2+(aq) + 2 e- Pb(s) -0.13 V Fe(s) Salt bridge 1 M Fe2+ 1 M Pb2+ -0.45 V The better oxidizing Pb2+ agent is:___ Pb2+actually gets So ___ reduced, and thus electrons flow to the left __ side, which _____ must be the ____ode. cath So the Pb electrode must be ______ive posit V V - -0.45 E°cell = -0.13 _____ _____ +0.32 V = _______ 28 Nernst Equation • See last week’s pink lab handout (Voltaic Cells), board, and below – Start with DG = DG + RTlnQ and substitute in DG = -nFEcell and DG = -nFEcell After some algebra (and substituting in values for R, assuming T = 298 K, and converting to a base 10 log): Ecell = Ecell o o 0.0592 V log Q n (T = 25C) 29 Standard vs. Nonstandard Cell Zn + Cu2+ Zn2+ + Cu; Q = ?? Recall lab—adding NH3 to Cu2+ side! Ecell = Ecell o o 0.0592 V log Q n (T = 25C) **Always write out the balanced redox equation before using the Nernst Equation or predicting whether Ecell should increase or decrease.** 30 Explain in detail an in a conceptual way why the cell potential goes up when the NH3 is added. Is the driving force for the cell rxn greater or smaller after the NH3 is added? 31 Relationship between variables (at any conditions; Mines Fig) DG = DG + RT ln Q Q Ecell = Ecell o o 0.0592 V log Q n (T = 25C) 32 Relationship between variables (at standard state conditions; From Tro) (T = 25C) 33 EXAMPLE 18.8 Calculating Ecell Under Nonstandard Conditions Determine the cell potential for an electrochemical cell based on the following two half-reactions: Oxidation: Reduction: E°cell = E°red(cat) - E°red(an) OR E°cell = E°red(cat) + E°ox(an) **Need to write balanced equation before E o = E o 0.0592 V log Q cell cell using Nernst! What will “n” be here?** n 34 35 36 37 38 39 40 41 42 43 Fig. 18.22 44 45 46 47 What mass of gold is plated in 25 minutes if the current is 5.5 A? Au3+ (aq) + 3 e- Au(s) 48 49 50