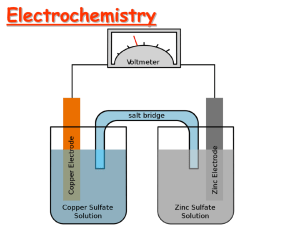
ELECTROCHEMISTRY Chapter 21
advertisement

ELECTROCHEMISTRY Chapter 21 • redox reactions • electrochemical cells • electrode processes • construction • notation Electric automobile • cell potential and Go • standard reduction potentials (Eo) • non-equilibrium conditions (Q) • batteries • corrosion 17-Nov-97 Electrochemistry (Ch. 21) 1 TRANSFER REACTIONS Atom transfer HCl (g) + H2O (l) Cl- (aq) + H3O+ (aq) Electron transfer Cu(s) + 2 Ag+(aq) Cu2+(aq) + 2 Ag(s) - 2 e2 x +1 e- Loss of Electrons = OXIDATION (LEO) 17-Nov-97 Gain of Electrons = REDUCTION (GER) Electrochemistry (Ch. 21) 2 Electron Transfer Reactions • Electron transfer reactions are oxidationreduction or redox reactions. • Redox reactions can result in : – generation of an electric current, or – be caused by imposing an electric current. • When external electric current is involved, this field of chemistry is called ELECTROCHEMISTRY. 17-Nov-97 Electrochemistry (Ch. 21) 3 Terminology for Redox Reactions • OXIDATION—loss of electron(s) by a species; increase in oxidation number. • REDUCTION—gain of electron(s); decrease in oxidation number. • OXIDIZING AGENT—electron acceptor; species is reduced. • REDUCING AGENT—electron donor; species is oxidized. 17-Nov-97 Electrochemistry (Ch. 21) 4 Direct Redox Reactions Oxidizing and reducing agents in direct contact. Cu(s) + 2 Ag+(aq) Cu2+(aq) + 2 Ag(s) 2Al (s) + 3Cu2+ 2 Al3+ + 3 Cu (s) 11_CuAg.mov 21mo2an1.mov 17-Nov-97 Electrochemistry (Ch. 21) 5 Indirect Redox Reactions A battery functions by transferring electrons through an external wire from the reducing agent to the oxidizing agent. Electron transfer Reduction Oxidation 11_battry.mov 21mo2an2.mov 17-Nov-97 Ions Electrochemistry (Ch. 21) 6 Balancing Equations Cu (s) + Ag+ (aq) Cu2+ (aq) + Ag (s) How to balance for both charge and mass ? Step 1: Identify the oxidation and reduction HALF-REACTIONS: RED: Ag+ + e- Ag OX: Cu Cu2+ + 2eStep 2: Balance each HALF-REACTION for charge and mass (done) Step 3:Multiply each half-reaction by a factor that makes the reducing agent supply as many electrons as the oxidizing agent requires - ELECTRON TRANSFER NUMBER (2 here) Oxidation (Reducing agent) Reduction (Oxidizing agent) Cu Cu2+ + 2e2 Ag+ + 2 e- 2 Ag Step 4:Add half-reactions to give the overall equation. Cu (s) + 2 Ag+ (aq) 17-Nov-97 Cu2+ (aq) + 2Ag (s) Electrochemistry (Ch. 21) 7 Balancing Equations (2) Balance the following in acid solution— VO2+ + Zn VO2+ + Zn2+ Step 1: Write the half-reactions Ox Zn Zn2+ Red VO2+ VO2+ Step 2: Balance each half-reaction for mass. Ox Zn Zn2+ Red 2 H+ + VO2+ VO2+ + H2O Add H2O on O-deficient side and add H+ on other side for H-balance. 17-Nov-97 Electrochemistry (Ch. 21) 8 Balancing Equations (3) Step 3: Balance half-reactions for charge. Ox Zn Zn2+ + 2eRed e- + 2 H+ + VO2+ VO2+ + H2O Step 4: Multiply by an appropriate factor to balance the electron transfer in OX. and RED. Ox Zn Zn2+ + 2eRed 2e- + 4 H+ + 2 VO2+ 2 VO2+ + 2 H2O Step 5: Add half-reactions Zn + 4 H+ + 2 VO2+ Zn2+ + 2 VO2+ + 2 H2O 17-Nov-97 Electrochemistry (Ch. 21) 9 Tips on Balancing Equations • Never add O2, O atoms, or O2- to balance oxygen. • Never add H2 or H atoms to balance hydrogen. • Be sure to write the correct charges on all the ions. • Check your work at the end to make sure mass and charge are balanced. 17-Nov-97 Electrochemistry (Ch. 21) 10 Why Study Electrochemistry? • Batteries • Corrosion • Industrial production of chemicals such as Cl2, NaOH, F2 and l • Biological redox reactions The heme group 17-Nov-97 Electrochemistry (Ch. 21) 11 Electrochemical Cells • An apparatus in which a redox reaction occurs by transferring electrons through an external connector. • VOLTAIC CELL • Product favored reaction chemical reaction electric current • ELECTROLYTIC CELL • Reactant favored reaction electric current chemical reaction 17-Nov-97 Electrochemistry (Ch. 21) Batteries are voltaic cells 12 CHEMICAL CHANGE ELECTRIC CURRENT Zn metal Cu2+ ions With time, Cu plates out onto Zn metal strip, and Zn strip “disappears.” • Zn is oxidized and is the reducing agent Zn(s) Zn2+(aq) + 2e• Cu2+ is reduced and is the oxidizing agent Cu2+(aq) + 2e- Cu(s) 17-Nov-97 Electrochemistry (Ch. 21) 13 CHEMICAL CHANGE ELECTRIC CURRENT (2) Oxidation: Zn(s) Zn2+(aq) + 2eReduction: Cu2+(aq) + 2e- Cu(s) -------------------------------------------------------Cu2+(aq) + Zn(s) Zn2+(aq) + Cu(s) Zn metal Cu2+ ions 17-Nov-97 Electrons are transferred from Zn to Cu2+, but there is no useful electric current. Electrochemistry (Ch. 21) 14 CHEMICAL CHANGE ELECTRIC CURRENT (2) • To obtain a useful current, we separate the oxidizing and reducing agents so that electron transfer occurs thru an external wire. wire elect rons Zn salt bridge Zn2+ ions Cu Cu2+ ions • This is accomplished in a VOLTAIC cell. (also called GALVANIC cell) • A group of such cells is called a battery. 17-Nov-97 Electrochemistry (Ch. 21) 15 wire ANODE CATHODE elect rons OXIDATION Zn Zn2+ ions REDUCTION Cu salt bridge Cu2+ ions • Electrons travel thru external wire. • Salt bridge allows anions and cations to move between electrode compartments. • This maintains electrical neutrality. 17-Nov-97 Electrochemistry (Ch. 21) 16 Electrons move from anode to cathode in the wire. Anions & cations move through the salt bridge. Electrochemical Cell 1!_cell.mov 21mo4an1.mov 17-Nov-97 Electrochemistry (Ch. 21) 17 Standard Notation for Electrochemical Cells Phase boundary ANODE Salt bridge Phase boundary Zn / Zn2+ // Cu2+ / Cu Anode electrode Cathode electrode Active electrolyte in oxidation half-reaction OXIDATION 17-Nov-97 CATHODE Active electrolyte in reduction half-reaction REDUCTION Electrochemistry (Ch. 21) 18 CELL POTENTIAL, E Zn Zn2+ + 2eANODE 2e- + Cu2+ Cu CATHODE • Electrons are “driven” from anode to cathode by an electromotive force or emf. • For Zn/Cu cell, this is indicated by a voltage of 1.10 V at 25C and when [Zn2+] and [Cu2+] = 1.0 M. 17-Nov-97 Electrochemistry (Ch. 21) 19 CELL POTENTIAL, Eo For Zn/Cu, voltage is 1.10 V at 25C and when [Zn2+] and [Cu2+] = 1.0 M. • This is the STANDARD CELL POTENTIAL, Eo • Eo is a quantitative measure of the tendency of reactants to proceed to products when all are in their standard states at 25 C. 17-Nov-97 Electrochemistry (Ch. 21) 20 o E and o G Eo is related to Go, the free energy change for the reaction. Go = - n F Eo • F = Faraday constant = 9.6485 x 104 J/V•mol •n = the number of moles of electrons transferred. Zn / Zn2+ // Cu2+ / Cu n for Zn/Cu cell ? 17-Nov-97 n=2 Electrochemistry (Ch. 21) Michael Faraday 1791-1867 Discoverer of • electrolysis • magnetic props. of matter • electromagnetic induction • benzene and other organic chemicals 21 Eo and Go (2) Go = - n F Eo • For a product-favored reaction – battery or voltaic cell: Chemistry electric current Reactants Products Go < 0 and so Eo > 0 (Eo is positive) • For a reactant-favored reaction - electrolysis cell: Electric current chemistry Reactants Products Go > 0 and so Eo < 0 (Eo is negative) 17-Nov-97 Electrochemistry (Ch. 21) 22 Calculating Cell Voltage • Balanced half-reactions can be added together to get the overall, balanced equation. I2 + 2e- Anode: 2 I- Cathode: 2 H2O + 2e- 2 OH- + H2 Net rxn: 2 I- + 2 H2O I2 + 2 OH- + H2 • If we know Eo for each half-reaction, we can calculate Eo for the net reaction. 17-Nov-97 Electrochemistry (Ch. 21) 23 STANDARD CELL POTENTIALS, Eo • Can’t measure half- reaction Eo directly. Therefore, measure it relative to a standard HALF CELL: the Standard Hydrogen Electrode (SHE). 2 H+(aq, 1 M) + 2eEo = 0.0 V 17-Nov-97 Electrochemistry (Ch. 21) H2(g, 1 atm) 24 Zn/Zn2+ versus H+/H2 Volts Zn - + Salt Bridge H2 H+ Zn2+ Zn Zn2+ + 2eOXIDATION ANODE 2 H+ + 2eH2 REDUCTION CATHODE Zn/Zn2+ half-cell combined with a SHE. Eo for the cell is +0.76 V 17-Nov-97 Electrochemistry (Ch. 21) 25 Eo for Zn/Zn2+ half-cell Volts Zn Overall reaction is reduction of H+ by Zn metal. - + Salt Bridge H2 H+ Zn2+ Zn Zn2+ + 2eOXIDATION ANODE Zn(s) + 2 H+ (aq) Zn2+ + H2(g) 2 H+ + 2eH2 REDUCTION CATHODE Eo = +0.76 V Therefore, +0.76 V. Eo for Zn Zn2+ (aq) + 2e- is ?? 17-Nov-97 Electrochemistry (Ch. 21) 26 Standard REDUCTION potentials Zn Zn2+ (aq) + 2eQ. A. Eo = +0.76 V Relative to H2 is Zn a (better/worse) reducing agent ? Zn is a better reducing agent than H2. What is Eo for the reverse reaction ? Zn2+ + 2e- Zn The value for the REDUCTION 1/2-cell is the negative of that for the OXIDATION 1/2-cell: Zn Zn2+ (aq) + 2e- Eo = +0.76 V THUS Zn2+ + 2e- Zn 17-Nov-97 Eo = -0.76 V Electrochemistry (Ch. 21) 27 Cu/Cu2+ and H2/H+ Cell Eo = +0.34 V Volts Cu + Salt Bridge H2 H+ Cu2+ Cu2+ + 2eCu REDUCTION CATHODE 17-Nov-97 H2 2 H+ + 2eOXIDATION ANODE Electrochemistry (Ch. 21) 28 Cu/Cu2+ half cell Eo Volts Cu + Salt Bridge H2 Overall reaction is reduction of Cu2+ by H2 gas. Cu2+ Cu2+ + 2eCu REDUCTION CATHODE H+ H2 2 H+ + 2eOXIDATION ANODE • Cu2+ (aq) + H2(g) Cu(s) + 2 H+(aq) • Measured Eo = +0.34 V +0.34 V • Therefore, Eo for Cu2+ + 2e- Cu is ?? 17-Nov-97 Electrochemistry (Ch. 21) 29 Zn/Cu Electrochemical Cell What is Eo for the Zn/Cu cell (Daniel’s cell) ?? wire Anode, negative, source of electrons Anode: Cathode: Net: elect rons Zn Zn2+ ions Cathode, positive, sink for electrons Cu salt bridge Cu2+ ions Zn(s) Zn2+(aq) + 2eCu2+(aq) + 2e- Cu(s) Eo = +0.76 V Eo = +0.34 V Cu2+(aq) + Zn(s) Zn2+(aq) + Cu(s) Eo = Eo(anode) + Eo(cathode) = 0.76 + 0.34 = +1.10 V 17-Nov-97 Electrochemistry (Ch. 21) 30 Uses of Eo Values This shows we can a) decide on relative ability of elements to act as reducing agents (or oxidizing agents) b) assign a voltage to a half-reaction that wire reflects this ability. elect rons Zn Zn2+ ions 17-Nov-97 Electrochemistry (Ch. 21) salt bridge Cu Cu2+ ions 31 STANDARD REDUCTION POTENTIALS Oxidizing ability of ion Half-Reaction Cu2+ + 2e- Cu + 0.34 2 H+ + 2e- H2 0.00 Zn2+ + 2e- Zn -0.76 BEST Oxidizing agent Cu ? ?2+ BEST Reducing agent ?Zn ? 17-Nov-97 Eo (Volts) Reducing ability of element Electrochemistry (Ch. 21) 32 Standard Redox Potentials, Eo • Any substance on the right will reduce any substance higher than it on the left. • Zn can reduce H+ and Cu2+. • H2 can reduce Cu2+ but not Zn2+ • Cu cannot reduce H+ or Zn2+. oxidizing ability of ion Eo (V) Cu2+ + 2e- Cu +0.34 2 H+ + 2e- H2 0.00 Zn2+ + 2e- Zn -0.76 reducing ability of element Use tabulated reduction potentials to analyse spontaneity of ANY REDOX REACTION 17-Nov-97 Electrochemistry (Ch. 21) 33 Determining Eo for a Voltaic Cell Volts Cd Fe2+ Cd2+ Cd Cd2+ + 2eor Cd2+ + 2e- Cd 17-Nov-97 Fe Salt Bridge Fe Fe2+ + 2eor Fe2+ + 2e- Fe Electrochemistry (Ch. 21) 34 Eo for Fe/Cd Cell Volts Cd LHS species Fe Salt Bridge is better oxidizing agent Cd 2+ + 2e- Cd Fe 2+ + 2e- Fe Cd2+ -0.40 -0.44 Fe2+ RHS species is better reducing agent • Fe is a better reducing agent than Cd • Cd 2+ is a better oxidizing agent than Fe 2+ • Overall reaction as written is spontaneous: Fe + Cd 2+ Cd + Fe 2+ Eo = +0.04 V • The reverse reaction is not spontaneous: Cd + Fe 2+ Fe + Cd 2+ Eo = -0.04 V 17-Nov-97 Electrochemistry (Ch. 21) 35