PowerPoint - Thermochemistry - Heat Energy
advertisement

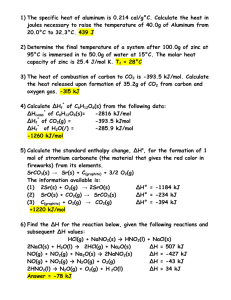
Hydrocarbons and Heat • Most hydrocarbons are used as fuels. • Knowing how much energy a fuel provides, can tell us if it is useful for a certain application. • For example, the amount of energy a food releases when burned, can tell us about it’s caloric content (fats release lots of energy). • Heat energy released during combustion can be measured with a calorimeter. • A “bomb calorimeter” is shown. It includes water in a heavily insulated container, a stirrer, valve, bomb chamber, ignition wires, & a thermometer (pg 577). Exothermic and Endothermic changes • An alternative to the bomb calorimeter is a “coffee cup” calorimeter, where two nested polystyrene cups take the place of the container • In either case, the change in heat of the water tells us about the reaction of the chemicals. • An increase in water temperature indicates that the chemicals released energy when they reacted. This is called an “exothermic” reaction. • In an “endothermic” reaction, water temperature decreases as the chemicals absorb energy. • We will see that heat is measured in Joules (J) or kiloJoules (kJ). Before we do any heat calculations, you should know several terms … Specific heat capacity balloon demo The heat needed to the temperature of 1 g of a substance by 1 C. Symbol: c, units: J/(gC). Heat capacity The heat needed to the temperature of an object by 1 C. Symbol: C (=c x m), units: J/C Heat of reaction The heat released during a chemical reaction. Symbol: none, units: J. Specific heat (of reaction) The heat released during a chemical reaction per gram of reactant. Symbol: h, units: J/g. Molar heat of reaction The heat released during a chemical reaction per mole of reactant. Symbol: H, units: J/mol. Heat Calculations • To determine the amount of heat a substance produces or absorbs we often use q = cmT • q: heat in J, c: specific heat capacity in J/(gC), m: mass in g, T: temperature change in C, • This equation makes sense if you consider units J J= x g x C gC For a list of c values see page 568 (table 3) Sample problem: (must know water = 4.18 J/gC) When 12 g of a food was burned in a calorimeter, the 100 mL of water in the calorimeter changed from 20C to 33C. Calculate the heat released. q=cmT = 4.18 J/(gC) x 100 g x 13C = 5.4 kJ More practice 1. 5 g of copper was heated from 20C to 80C. How much energy was used to heat the Cu? q=cmT = 0.38 J/(gC) x 5 g x 60 C = 114 J 2. If a 3.1 g ring is heated using 10.0 J, it’s temp. rises by 17.9C. Calculate the specific heat capacity of the ring. Is the ring pure gold? q=cmT 10.0 J c = q/mT= = 0.18 J/(g°C) 3.1 g x 17.9C The ring is not pure. Gold is 0.13 J/(gC) -pg. 568 3. Do questions 5, 6 on pg. 596 Do questions 7, 8 on pg. 570 5. q=cmT = 4.18 J/(gC) x 2570 g x 92 C = 988319 J = 988 kJ = 0.988 MJ 6. q=cmT 1 750 000 J T = q/cm = = 33.5°C 4.18 J/(gC) x 12500 g Since the temperature started at 5.0°C, the final temperature is 38.5°C. 7. q=cmT = 0.86 J/(gC) x 2500 g x 335C = 720 kJ or 0.72 MJ 8. Total heat = water heat + pot heat = 4.18 J/(gC) x 1200 g x 53.0C = 265 848 J = 0.510 J/(gC) x 450 g x 53.0C = 12163.5 J = 278 kJ Heat Capacity Calculations • Recall that heat capacity (J/C) is different from specific heat capacity (J/gC). • Heat capacity is sometimes a more useful value • For example, because a calorimeter includes wires, the stirrer, thermometer, etc. some heat will be transferred to these other materials. • Rather than having to calculate q for each material (like question 8) a J/C value is used. Sample problem: q=cmT A calorimeter has a heat capacity of 2.05 kJ/C. How much heat is released if the temperature change in the calorimeter is 11.6C? q = cm T q= 2.05 kJ/C x 11.6 C = 23.8 kJ Thermochemical Equations Thermochemical equations are chemical equations with an added heat term. • KBrO3(s) + 42 kJ KBrO3(aq) This is endothermic (heat is absorbed/used) • 2 Mg(s) + O2(g) 2 MgO(s) + 1200 kJ This is exothermic (heat is produced/released) Read 12.3 (pages 582 – 585). Do 2-6 (pg. 585). Sample problem: 3.00 g of octane was burned in a calorimeter with excess oxygen, the 1000 mL of water in the calorimeter rose from 23.0C to 57.6C. Write the thermochemical equation for octane, representing the molar heat of combustion. Page 585 2. Specific refers to mass in grams. 3. Specific heat of reaction: J/g or kJ/g, etc. Molar heat of reaction: J/mol or kJ/mol, etc. 4. J/mol = J/g x g/mol (molar mass is used) 5. Exothermic. E.g. CH4 + 2O2 CO2 + 2H2O + x kJ Other examples include propane, octane, Mg 6. 49.90 kJ 26.04 g = 1299 kJ/mol x g mol C2H2 + 2.5O2 2CO2 + H2O + 1299 kJ or 2C2H2 + 5O2 4CO2 + 2H2O + 2598 kJ Sample: First, calculate the heat released by the combustion reaction via q=cmT … = 4.18 J/(gC) x 1000 g x 34.6 C = 144 628 J Octane (C8H18) has a molar mass of 114.26 g/mol We can determine the molar heat of reaction 1) via the specific heat of reaction or 2) directly h = J = 144 628 J H = 48.2 kJ x 114.26 g g 3.00 g g mol = 48209 J/g = 48.2 kJ/g = 5508 kJ/mol or J 144 628 J = 5508 kJ/mol H = = mol 0.0263 mol C8H18 + 12.5O2 8CO2 + 9H2O + 5508 kJ For more lessons, visit www.chalkbored.com