PowerPoint Lesson - Dalton's Law of Partial Pressures
advertisement

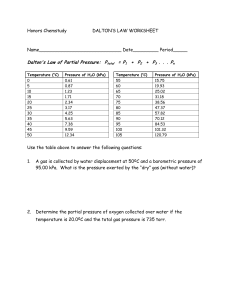
How Much Pressure? Q - A balloon is filled with pure oxygen. What is the pressure of the oxygen in the balloon? A - Atmospheric pressure. If it wasn’t, then the balloon would expand or shrink. Q - A windbag is blown up with exhaled air. What is the pressure of oxygen in the bag? A – Around 16 - 21% of atmospheric pressure (O2 is 16% of exhaled air, 21% of atmosphere) Q - A solid container is filled with pure oxygen. What is the pressure in the container? A - It could be anything. The container is solid and therefore cannot shrink or expand. Dalton’s law of partial pressures Read pages 460 - 461 1. In the 18th century what did many scientists believe about the earth’s atmosphere? 2. The % of which component of air varies the most? 3. Argon makes up about 0.93% of dry air. List the other 3 gases present in dry air (with their %s). Around what % of dry air is made up by gases other than these 4? 4. Give Dalton’s law of partial pressures & the equation. 5. 1 L of N2 at 50 kPa is mixed with 1 L of O2 at 60 kPa, to form a 1 L mixture of the gases. What is the resulting pressure? What are the partial pressures? 6. A balloon contains 75 kPa N2, 15 kPa O2, 5 kPa CO2, and water vapour. If atmospheric pressure is 100 kPa what is the partial pressure of water vapour? Dalton’s law of partial pressures 1. That the atmosphere was a single chemical compound. 2. Water 3. N2: 78.08%, O2: 20.95%, CO2: 0.04%. These add up to 100% of dry air, so there are almost no other gases (really, 0.002%). 4. The total pressure of a mixture of nonreacting gases is equal to the sum of the partial pressures of the individual gases (Ptotal = P1 + P2 + P3 + …) 5. Total pressure = 60 kPa + 50 kPa = 110 kPa 6. 100 - (75 + 15 + 5) = 100 - 95 = 5 kPa Summary Dalton found that the total pressure of mixed gases is equal to the sum of their individual pressures (provided the gases do not react). 50 kPa 100 kPa 150 kPa Note: all of these + = volumes are the 1 L oxygen 1 L nitrogen 1 L mixed gas same This works according to the KMT because at the same temperature molecules of different gases have the same Ek. It doesn’t matter if the molecules are O2 or H2. Both collide with the container or other molecules with the same force. Vapour Pressure Defined • Vapour pressure is the pressure exerted by a vapour. E.g. the H2O(g) in a sealed container. Eventually the air above the water is filled with vapour pushing down. As temperature , more molecules fill the air, and vapour pressure . • Yet, molecules both leave and join the surface, so vapour pressure also pushes molecules up. • To measure vapour pressure we can heat a sample of liquid on top of a column of Hg and see the pressure it exerts at different °C. Vapour pressure Measuring Vapour Pressure Vapour pressure for H2O °C kPa °C kPa 10 1.23 50 12.33 20 2.34 75 38.54 Temperature 30 4.17 100 101.3 See pg. 464 for more • When the vapour pressure is equal to the atmospheric pressure (Patm), the push out is enough to overcome Patm and boiling occurs. • Thus, water will boil at a temperature below 100 °C if the atmospheric pressure is reduced. Collecting gases over water • Many times gases are collected over H2O • Often we want to know the volume of dry gas at STP (useful for stoichiometry). For this we must make 3 corrections: 1. The level of water inside and outside the tube must be level (so pressure inside is equal to the pressure outside). 2. The water vapour pressure must be subtracted from the total pressure (to get the pressure of the dry gas). 3. Finally, values are converted to STP using the combined gas law. Sample calculation A gas was collected over 21°C H2O. After equalizing water levels, the volume was 325 mL. Give the volume of dry gas at STP (Patm=102.9 kPa). Step 1: Determine vapour pressure (pg. 464) At 21°C vapour pressure is 2.49 kPa Step 2: Calculate the pressure of dry gas Pgas = Patm - PH2O = 102.9 - 2.49 = 100.41 kPa Step 3: List all of the data T1 = 294 K, V1 = 325 mL, P1 = 100.41 kPa Step 4: Convert to STP = 299 mL (P1)(V1)(T2) (100.4 kPa)(325 mL)(273 K) V2= = (P2)(T1) (101.325 kPa)(294 K) Assignment 1. 37.8 mL of O2 is collected by the downward displacement of water at 24°C and an atmospheric pressure of 102.4 kPa. What is the volume of dry oxygen measured at STP? 2. Try questions 8 – 10 on page 465. 3. 236 mL of H2 is collected over water at 22°C and at an atmospheric pressure of 99.8 kPa. What is the volume of dry H2 at STP? 4. If H2 is collected over water at 22°C and an atmospheric pressure of 100.8 kPa, what is the partial pressure of the H2 when the water level inside the gas bottle is equal to the water level outside the bottle? 1) Vapor pressure at 24C = 2.98 kPa Pgas = Patm - Pvapor = 102.4 kPa - 2.98 kPa = 99.42 kPa = P1 V1 = 37.8 mL, P1 = 99.42 kPa, T1 = 297 K V2 = ?, P2 = 101.3 kPa, T2 = 273 K P1V1 = T1 (99.42 kPa)(37.8 mL) = (297 K) P2V2 T2 (101.3 kPa)(V2) (273 K) (99.42 kPa)(37.8 mL)(273 K) (V2) = = 34.1 mL (297 K)(101.3 kPa) 3) Vapor pressure at 22C = 2.64 kPa Pgas = Patm - Pvapor = 99.8 kPa - 2.64 kPa = 97.16 kPa = P1 V1 = 236 mL, P1 = 97.16 kPa, T1 = 295 K V2 = ?, P2 = 101.3 kPa, T2 = 273 K P1V1 P2V2 = T1 T2 (97.16 kPa)(236 mL) (101.3 kPa)(V2) = (295 K) (273 K) (97.16 kPa)(236 mL)(273 K) (V2) = = 209 mL (295 K)(101.3 kPa) Answers 4 - Total pressure = PH2 + PH2O 100.8 kPa = PH2 + 2.64 kPa 100.8 kPa - 2.64 kPa = PH2 = 98.16 kPa For more lessons, visit www.chalkbored.com