Wittmers.Body_Compartments
advertisement
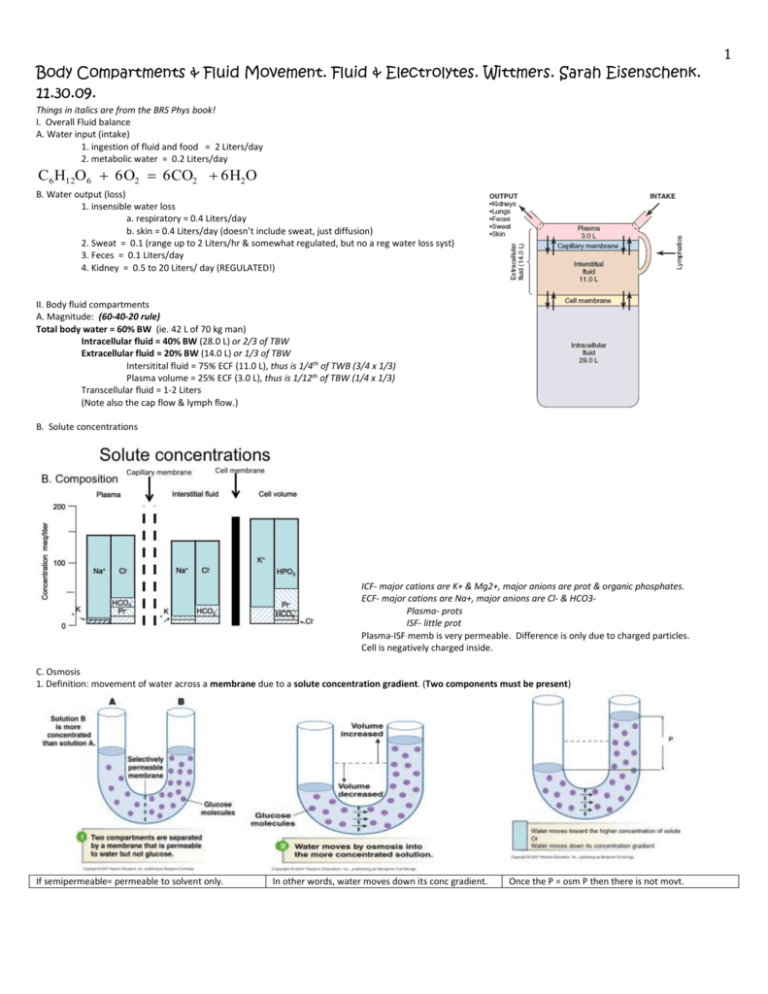
Body Compartments & Fluid Movement. Fluid & Electrolytes. Wittmers. Sarah Eisenschenk. 11.30.09. Things in italics are from the BRS Phys book! I. Overall Fluid balance A. Water input (intake) 1. ingestion of fluid and food = 2 Liters/day 2. metabolic water = 0.2 Liters/day C6 H12O6 6O2 6CO2 6H2O B. Water output (loss) 1. insensible water loss a. respiratory = 0.4 Liters/day b. skin = 0.4 Liters/day (doesn’t include sweat, just diffusion) 2. Sweat = 0.1 (range up to 2 Liters/hr & somewhat regulated, but no a reg water loss syst) 3. Feces = 0.1 Liters/day 4. Kidney = 0.5 to 20 Liters/ day (REGULATED!) II. Body fluid compartments A. Magnitude: (60-40-20 rule) Total body water = 60% BW (ie. 42 L of 70 kg man) Intracellular fluid = 40% BW (28.0 L) or 2/3 of TBW Extracellular fluid = 20% BW (14.0 L) or 1/3 of TBW Intersitital fluid = 75% ECF (11.0 L), thus is 1/4th of TWB (3/4 x 1/3) Plasma volume = 25% ECF (3.0 L), thus is 1/12th of TBW (1/4 x 1/3) Transcellular fluid = 1-2 Liters (Note also the cap flow & lymph flow.) B. Solute concentrations ICF- major cations are K+ & Mg2+, major anions are prot & organic phosphates. ECF- major cations are Na+, major anions are Cl- & HCO3Plasma- prots ISF- little prot Plasma-ISF memb is very permeable. Difference is only due to charged particles. Cell is negatively charged inside. C. Osmosis 1. Definition: movement of water across a membrane due to a solute concentration gradient. (Two components must be present) If semipermeable= permeable to solvent only. In other words, water moves down its conc gradient. Once the P = osm P then there is not movt. 1 2 Impt to note that gives osmotic P in atm. Refer to molar conc of the particles, in fact can say it is proportional to osm P and forget about RT. Once piston P is = osmotic P, then Jv=0 (water movt) Note: 0.6 osmols = 600 milliosmoles 2. 0.3 x 2 particles = 0.6 osmols Osmosis and a leaky membrane theoretical actual actual reflectioncoefficient theoretical is unique for each molecule and membrane system If theoretical osm P>actual osm P than memb is NOT semi-permeable. So, must define the reflection coef, which gives an idea of osmotic activity for a molecule of a given memb. : Close to 1 = 0 perm, while < 1=inc perm. Only talking about solutions here! No membs or cells! However Solution A is usually cell. given Hypertonic solution contains a greater conc of impermeable solutes than the soln on the other side of the memb (CELL). (SHRINKS LIKE TOP) Hypotonic solution contains a lesser conc of impermeable solutes than the soln on the other side of the memb (CELL). (SWELLS LIKE BOTTOM). Tonicity= rxn of cell to the soln. Osmolarity does not equal tonicity. inside of a 3 See Sketch below. In this scenario, the rat rbcs are in hypotonic (SWELLS) and hyposmotic solution. This direct relationship is not always the case. In this example, there are fxnal changes: the rbcs start as biconcave discs. Once swellsphere. Max volv change of the rbc is at 150 mosm. Urea is permeable to cell memb, so 300 mosm NaCl ICF and 300 mosm ECF (isosmotic), but the urea enters the cell lysis as water follows. (hypotonic) 300 mosm urea + 300 mosm NaCl is hyperosmotic compared to 300 mOsm of ICF. But urea crosses memb, preventing shrinkage of cellisotonic soln. Gave ethanol IV for methanol poisoning hemolysis of rbcs (red urine), this was like giving urea. Could have added glucose or NaCl (imperm) making the solution slightly hyperosmotic w/o lysis. Easier 4 solution is to give the guy glass of alcohol. Note: Look at osmolarity prior to water movt to determine hyper vs hypo, then look at tonicity once particles/water move across memb. Fluid and osmotic changes with pathology and treatment. Review of normal state: 70 kg subject (ICF =40%,ECF=20%,TBW=60%) volume Con (mosm) Total sol ECF 14 300 4,200 ICF 28 300 8,400 TBW 42 300 12,600 AKA. “Isosmotic vol expansion” ECF Vol incs, but no change in osm bc NaCl is imperm, no water shift (so no change in ICF vol). Plasma prot & hematocrit dec, RBCs do not change size. Arterial BP incs. Iv. infusion of 3% NaCl solution Solution analysis of particle concentration 3% NaCl = 3g/100ml = 30g/L NaCl = 58 g/mole molar cconcentration = 30/58= 0.517molar osmolar concentration = 1.035 osmolar If you give 2L of the solution this is equivalent to 2.07 moles of particles or 2,070 milliosmoles Hyperosmotic soln incs ECF vol & Conc. Don’t Conc osm has to equilibrate btwn compartments, so 333 mosm. even look at TBW yet. Here can find the Qualitative answer of how far water went can be found here. direction water will go (qualitative answer), 14,670/333=44. Hypertonic soln, yet hypertonic. ICF decd (SHRINK) into the ECF. Aka “Hyperosmotic volume expansion”. Osmolarity of ECF incs bc osmoles have been added to ECF. Water shifts from ICF to ECFinc of ICF osm until it equals ECF osmincd ECF Vol & dec ICF Vol. Plasma prot & hematocrit dec. ~ to administration of urea. Incd ECF, decs conc osmol, Solutes stay the same bc didn’t add any. Water flows into cells. (Hypoosmotic) Conc osm has to equilibrate btwn compartments, so 286 mosm. Know that ICF increased (so did ECF slightly), so hypotonic (SWELL) 5 AKA “Hyposmotic volume expansion”. Osmolarity of ECF decs. ECF vol incsICF osm decsincd ICF Vol. Plasma prot conc decs. Hematocrit unchanged (water shifts to RBCs, incg their vol & offsetting the diluting effect of the gain of ECF vol.) Problem in dehydration *Presenting information: Your patient has not been able to drink for the past two days. No vomiting or diarrhea was reported; however, she has been doing some moderate exercise. Shows signs of sever dehydration. *Data: body build average, weight 153 lbs. with a plasma osmolarity of 340 mosmoles. *How much fluid would you have to replace to repair the deficit? Convert body weight 153 lbs./2.54lbs.kg-1 = 60 kg volume Con (mosm) Total sol ECF 12 340 4,080 ICF 24 340 8,160 TBW 36 340 12,240 First, find the volume of TBW needed to return to Con of 300 mosom. Manipulate this equation: conc=solutes/Vol. Next solve for the vol difference. Then solve for the other volumes (ECF & ICF), using vol=solutes/conc. Calculations Make the assumption that no salt is lost What would the total body water have to be to get back to 300 mosmols/liter Volume = 12240 mosmols/ 300mosmols/L = 40.8 L Vol replaced = 40.8-36 = 4.8 L To return the plasma osmolarity to 300 mosm/liter Questions: 1. When would a cell swell in a hypertonic or hypotonic solution? 2. In order for osmosis to occur what two components must be present? 3. T/F: If theoretical osm P>actual osm P than memb is NOT semi-permeable. 4. Given the following scenario: A rbc is in a solution of 300 mosm urea + 300 mosm NaCl, would the solution be considered hyper, iso, or hypo osmotic? Hyper, iso, or hypo tonic? 5. Which way will the water move in this scenario w/ infusion of 3% NaCl? 6 Answers: 1. Hypotonic solution. 2.A semi-permeable membrane & solute concentration gradient. 3. T 4. Hyperosmotic. & isotonic. 5. Into the ECF.







