nitro
advertisement

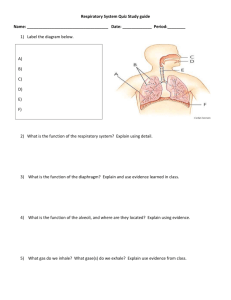
Kenneth Frederick How the Overuse of Fertilizer has Created Multiple Ecosystem Toxins Significance of this Topic • • • There has been a growing consensus among the mainstream population that global warming and increased carbon dioxide levels are a serious global threat. However, there has not been as much discussion about the global nitrogen cycle that has become dysfunctional. I wanted to bring attention to a topic that is very important and needs to be considered a real threat to the biosphere. Background Information • Fertilizer synthesis began with a simple process • N2+3H2 becomes 2NH3 • This is called the Haber-Bosch Process • Atmospheric N2 is biologically unavailable, so this reaction makes nitrogen biochemically available for use among organisms. Background continued • With an increasing global population, food production had to obviously increase. • Fertilizer allows for greater nutrient intake among plants which allow for higher crop yields. • To match food production demands, fertilizer use has risen dramatically. Ecosystem Pollutants Caused from Inefficient Fertilizer Use • With a higher ratio of nitrified compounds available compared to denitrified compounds that become N2 again, this has caused air, water, and ecological pollutants. • The most common nitrogen pollutant are nitrates. Nitrogen Compounds as Air Pollutants • Consistent inhalation of nitrites can cause serious damage to the respiratory system • Nitrous oxide causes increases in low lying ozone • At high altitudes, nitrous oxide destroys ozone • Nitrous oxide is a powerful greenhouse gas which absorbs 200 times the radiation compared to carbon dioxide. Nitrite Inhalation • Long-term exposure to nitrites can injure epithelial cells of the bronchioles and alveoli along with alveolar macrophages. • It decreases ciliate epithelium because of the body’s inflammatory response • Epithelial and macrophage loss in the lungs increases the risk for obtaining infections or fibrosis The Body’s Response to Respiratory Inflammation • The inflammation caused by nitrite inhalation leads to epithelial proliferation • The cells increase in their growth and division to compensate for injured cells • This process leads to hyperplasia which is an increase in organic tissue Other Adverse Effects • The respiratory system will produce the protein collagen to strengthen the damaged connective tissue • Fluid can build up in the lungs leading to impairment in breathing and even respiratory failure Mechanism for Epithelial Damage • Once in contact with respiratory epithelium cells, nitrites are converted to free radicals which causes oxidative stress in the airways. These free radicals are not destroyed by anti-oxidants so inflammatory cells and mediators are released. Ex. cytokines and chemokines • The free radicals react with lipids causing lipid-peroxidation. Lipid Peroxidation • This process is the oxidative degradation of lipids. • Free radicals take electrons from lipids in cell membranes causing cell damage • Generally, this affects polyunsaturated fatty acids which can lead to cell damage in the lungs Low Lying Ozone • Elevated levels of atmospheric nitrous oxide will increase low lying ozone concentrations • This can cause inflammation of the respiratory airway • The danger is greater for those who have asthma or chronic lung diseases Mechanism for Ozone Attack • Ozone is a powerful oxidizing agent that causes increased neutrophilic activity upon entering the respiratory system • Pre-inflammatory cytokines, albumin, lactate dehydrogenase in bronceoalveolar secretions are the body’s immune response to ozone • This causes inflammatory damage to the epithelial cells in the lungs which increases the risk to obtain respiratory pathogens • Bronchial mucosal biopsies confirmed the inflammatory response to ozone Inflammatory Response Effect • This inflammatory response is part of the hyperactivity that occurs in the respiratory pathway • This causes epithelial damage and increases the permeability of endothelium and epithelium which increases the risk for infections in the respiratory system Pig Study • In a study of ozone inhalation of guinea pigs, one day after inhalation, respiratory pathways became hyperactive because of the release of eosinophil basic major protein that inhibits neuronal muscarinic receptors, causing acetylcholine release and increased smooth muscle activity. Nitrates as a Water Pollutant • When water is consumed containing nitrates, gut microbes reduce nitrates to nitrites. Nitrites then compete with oxygen to bind to hemoglobin. • Hemoglobin is converted to methemoglobin which cannot bind to oxygen thereby decreasing oxygen transportation to tissues throughout the body • This is called methemoglobinemia or Blue Baby Syndrome • This process is dangerous for infants because the lack of oxygen in the blood can lead to brain damage or even death. Mechanism for Methemoglobinemia • When gut bacteria reduce nitrates to nitrites, nitrites oxidize ferrous(Fe2t) iron to ferric(Fe3t) iron in hemoglobin which converts hemoglobin to methemoglobin. Oxygen cannot bind to the methemoglobin site but it can bind to the other heme sites that are still Fe2t within the same tetrameric hemoglobin unit. • This leads to an increased affinity for oxygen to bind to these subunits. There is a decrease in the ability for red blood cells to release oxygen to tissues. This is called hypoxia. Other Risks from Nitrates in Water • Nitrate can competitively inhibit iodine uptake and induce hypertrophic changes in the thyroid gland • Damage to this area can affect hormonal regulation in the bloodstream which will adversely affect metabolic activity • Nitrates react in the stomach to form nitrosating agents which react with amines and amides from proteins to form N-Nitroso compounds which can be human carcinogens Adverse Effects on the Environment from Bioavailable Nitrogen • Having more nitrified nitrogen in water, soil, and the atmosphere affects other biotic factors of the ecosystem as well as abiotic factors • There is an increase in nitrogen acid rain which decreases the pH of soil and aquatic environments • The increased acidity decreases buffering capacity which can cause water corrosivity towards lead and copper. How Plants are Affected • Plant diversity decreases as a result of nutrient increases in the environment • Plants that have been used to growing in low nitrogen concentrations will be outcompeted by plants that require more nutrients. • Losing plant diversity will negatively affect the entire ecosystem Eutrophication’s Effect on Microbial Activity • • • • With an increase in bioavailable nutrients, this will cause an increase in primary producers With more primary producers in the environment, this creates more resources for primary consumers and organisms that have symbiotic relationships with them. This positively affects pathogenic organisms in the ecosystem too. With more intermediate hosts there will elevated levels of parasitic activity. Ex. Cholera, Mosquitoes that carry West Nile, Malaria, and Encephalitis Solutions to this Problem • The most important thing that can be done to balance the nitrogen cycle is for fertilizers to be used more efficiently. Currently, there is far too much fertilizer used unnecessarily. Many times, the extra use doesn’t even improve crop yields. • Increased regulations can be implemented to ensure protocols are followed • Less meat consumption among people will lead to a decrease in fertilizer used to grow food used to feed animals References • • • • • • • • • Arbex, Marcos et al. Air pollution and the respiratory system. Jornal Brasileiro de Pneumologia. October 2012. Vol. 38, No.5: 643-655. Fields, Scott. Global nitrogen: cycling out of control. Environmental Health Perspective. July 2004. Vol. 112, No. 10: 556-563. Filippidou, E.C. and Koukouliata, A. Ozone effects on the respiratory system. Progress in Health Sciences. 2011. Vol. 1, No. 2: 144-155. McKenzie, Valerie and Townsend, Alan. Parasitic and infectious disease responses to changing global nutrient cycles. Ecohealth. 2007. Vol. 4: 384-396. Persinger, Rebecca et al. Molecular mechanisms of nitrous dioxide induced epithelial injury in the lung. Molecular and Cellular Biochemistry. 2002. Vol. 234: 71-80. Ward, Mary. Too much of a good thing? Nitrate from nitrogen fertilizers and cancer. Rev Environ Health. October 2009. Vol. 24, No. 4: 357-363. Weinberger, Barry et al. The toxicology of inhaled from nitric oxide. Toxicological Sciences. 2000. Vol. 59, No. 1: 5-16. http://www.epa.gov/ogwdw/disinfection/tcr/pdfs/whitepaper_tcr_nitrification.pdf http://www.who.int/water_sanitation_health/dwq/chemicals/nitratenitrite2ndadd.pdf