3. GPCRs
advertisement

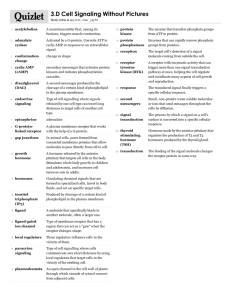
MEMBRANE PROTEINS Anna Adell, Mariona Bartrolí, Marina Brasó i Abel Eraso Index 1. Introduction • What is cell membrane? • Integral Proteins • Crystallization 2. Transmembrane Proteins • β - Barrels • α-Helical 3. GPCRs • What are G protein – coupled receptors? • Classification • Sequence conservation • Class A • SCOP • Chronology • Alignments • β2-Adrenergic Receptor • Extracellular Loop 2 (ECL2) • Ligand binding site • Ionic Lock • Activation 4. 5. Conclusions Bibliography 1. Introduction What is cell membrane ? Composed of a phospholipid bilayer with a collage of many different proteins, lipids and carbohydrates It has many functions Membrane Proteins: They can be classified into two main categories: peripheral and integral proteins Fig2. Cell membrane with integral proteins embedded. Fig1. Cell membrane with peripheral proteins. 1. Introduction Transmembrane proteins Extracellular region Transmembrane region Intracellular region Fig3. Cell membrane with a transmembrane protein. Domain Functions Extracellular Involved in cell – cell signaling Let interactions with other molecules Transmembrane Forms channels and pores Intracellular Activates intracellular signaling pathways 1. Introduction CRYSTALLIZATION. Why is it so complicated? Difficulties in handling and crystallization of integral membrane proteins reside in the amphiphatic nature of their surface They have hydrophobic transmembrane regions and two hydrophillic regions, one of each side of the membrane As a result, membrane proteins are not soluble in aqueous buffers or in organic solvents Membrane protein structural biology: The most challenging targets in structural biology 1. Introduction CRYSTALLIZATION. Why is it so complicated? Trying to solubilize and crystalize transmembrane proteins: To solubilize transmembrane proteins is necessary to add amphipathic molecules such as detergents to cover the transmembrane region Addition of detergents doesn’t guarantee stably solubilized membrane proteins Nowadays, crystallization techniques try to add lipids to the detergents to improve the solubilization process Index 1. Introduction • What is cell membrane? • Integral Proteins • Crystallization 2. Transmembrane Proteins • β - Barrels • α-Helical 3. GPCRs • What are G protein – coupled receptors? • Classification • Sequence conservation • Class A • SCOP • Chronology • Alignments • β2-Adrenergic Receptor • Extracellular Loop 2 (ECL2) • Ligand binding site • Ionic Lock • Activation 4. 5. Conclusions Bibliography 2. Types of transmembrane proteins β - Barrels • Are found in the outer membranes of Gram – negative bacteria • It is formed by an array of beta – strands arranged in an antiparallel manner Extracellular Extracellular Intracellular Fig4. NalP protein Fig6. Fig5. OmpA protein Fig7. 2. Types of transmembrane proteins α - Helical Extracellular Intracellular Fig8. PufX protein Bitopic Polytopic Fig9. Rodhopsin • It contains a hydrophilic domain on each side of the membrane • It contains a multiple hydrophilic domains on both sides of the membrane Fig10. Bitopic proteins and Polytopic proteins Index 1. Introduction • What is cell membrane? • Integral Proteins • Crystallization 2. Transmembrane Proteins • β - Barrels • α-Helical 3. GPCRs • What are G protein – coupled receptors? • Classification • Sequence conservation • Class A • SCOP • Chronology • Alignments • β2-Adrenergic Receptor • Extracellular Loop 2 (ECL2) • Ligand binding site • Ionic Lock • Activation 4. 5. Conclusions Bibliography 3. GPCRs What are G protein-coupled receptors ? Integral membrane proteins are characterized by seven α-helix transmembrane domains with an extracellular N terminus and a cytoplasmastic C terminus Mediate the effect of numerous ligands Activate different signaling paths The most important family of membrane proteins One of the most important types of receptors GPCR 3. GPCRs Classification Fig12. Scheme of structure of the class B Fig11. Scheme of structure of the class A Fig13. Scheme of structure of the class C 3. GPCRs Sequence Conservation A_adenosine A_beta-1 A_beta A_rhodopsin B_Glucagon B_Pituitary B_Parathyroid B_Vasoactive C_Gamma-aminob C_Metabotropic C_Metabotropic C_Taste P..FAI..TI.........---.........STGFCA..ACHGCLF.IACFVLVLTQSSIFSLLA..IA..IDRY..IA.....I--RIPL-RY-NGLVT P..FGA..TI.........VVW.........GRW-EY..GSFFCEL.WTSVDVLCVTASIETLCV..IA..LDRY..LA.....I--TSPF-RY-QSLLT P..FGA..AH.........ILM.........KMW-TF..GNFWCEF.WTSIDVLCVTASIETLCV..IA..VDRY..FA.....I--TSPF-KY-QSLLT T..STL..YT.........SLH.........GYF-VF..GPTGCNL.EGFFATLGGEIALWSLVV..LA..IERY..VV.....VCKPMSN---F--RFG R..YSQ..KIg......ddLSV.........STW-LSdgAVAGCRV.AAVFMQYGIVANYCWLLVegLY..LHNL..LG.....L-ATLPERSFF----A..ISV..FI.........---.........KDWILY..AEQDSN-.HCFISTVECKAVMVFFHY..CV..VSNY..FW.....L-FIEGL--YLFTLLV Y..SGA..TLdeaerlteeELRaiaqappppATA-AA..GYAGCRVaVTFFLYFLATNYYWILVE..--..-GLY..LH.....SLIFMAF---FS---E IkdLAL..FD.........SGE.........SDQCSE..GSVGCKA.AMVFFQYCVMANFFWLLV..EG..LYLYtlLA.....V-SFFSERKYF----P..LGLdgYH.........IGR.........NQF---..-PFVCQ-.-ARLWLLGLGFSLG----..--..---Y..GS.....MFTKIWW---VHTVFT P..FTL..IA.........--K.........PTT---..--TSCYL.QRLLVGLSSAMCYSALVT..KTnrIARI..LAgskkkICTRKP-------RFM P..STA..VC.........TLR.........RLG---..--LGTAF.SVCYSALLTKTN------..-R..IARI..FG.....-GAREGA---QRPRFI P..TRP..AC.........LLR.........QALFAL..G-----F.TIFLSCLTVRS--FQLII..I-..----..FK.....FSTKVPTF--YHAWVQ A_adenosine A_beta-1 A_beta A_rhodopsin B_Glucagon B_Pituitary B_Parathyroid B_Vasoactive C_Gamma-aminob C_Metabotropic C_Metabotropic C_Taste GTRAKGIIAIC...WVL.SFAIG-.LTPM....LGW-N----NCGQPKEGKNHSQGCGEGQVAC.LF-EDV--.-----VPMNYMVYFN..FFA.CVLVP RARARGLVCTV...WAI.SALVSF.LPIL....MHWWRAESDEA--RR--CYNDPKC------C.D-F--VT-.------NRAY-AIAS..SVV.SFYVP KNKARVIILMV...WIV.SGLTSF.LPIQ....MHWYRATHQEA--IN--CYANETC------C.D-F--FT-.------NQAY-AIAS..SIV.SFYVP ENHAIMGVAFT...WVM.ALACAA.-PPL....AGWS-------------R---YIPEGLQCSC.GID--YYT.LKPEVNNESF-VIYM..FVV.HFTIP -----------...---.SLYLG-.----....IGW------GAPMLFVVPWAVVK-------C.LF-ENV-Q.CWTSNDNMGFWWILR..FPV.FLAIETFFPERRYFY...WYT.IIGWGT.PTVC....VTVW------A-TLR--LYFDDTG------CwDM-NDST-.------ALWW-VIKG..PVVgSIMVN KKYLWGFTVFG...WGLpAVFVAV.----....--WV---SVRA------TLANTG-------C.W-D--LS-.----SGNKKW-IIQV..PIL.ASIV----WGYILIG...WGV.PSTFT-.----....MVWT-----IA-RIH---FEDYG-------C.W-D---T-.-----INSSLWWIIKgpILT.SILVN KKEEKKEWRKTlepWKL.YATVGL.LVGMdvltLAIWQIVDPLHRTIE--TFAKEEP-----KE.DID--VSIlPQLEHCSSRKMNTWL..GIF.YGYKG SAWAQVIIA--...---.SILISVqLTLV....VTLI---------IM--E--PPMP-------.-IL--SY-.---PSIKEVYLICNT..SNL.GVVAP SPASQVAIC--...--L.ALISGQ.LLIV....VAWL---VVEA----PGTGKETAP-------.ER-REVV-.----TLRCNH-RDAS..MLG.SLAYN NHGAGLFVMIS...SAA.QLLIC-.LTWL....VVW---TPLPA---R--EYQR-FP------H.LVMLECT-.---ETNSLGF-----..-IL.AFLYN Fig14. Fragment of sequence alignment of the three main classes of GPCRs 3. GPCRs Class A Fig16. Scheme of structure of the class B Fig15. Scheme of structure of the class A Fig17. Scheme of structure of the class C 3. GPCRs Class A. SCOP Fig 18. Class A classification from SCOP (Structural Classification of Proteins) 3. GPCRs Class A. Chronology Before 2000 2000 2007 Rhodopsin Palczewski et al. Aug. 2000 2008 2008-2012 β1 -Adrenergic Receptor Warne et al. July 2008 2-Adenosine Receptor Jaakola et al. Oct. 2008 β2-Adrenergic Receptor Cherezov et al. Nov. 2007 D1-R H1-R SPS1 … 3. GPCRs adenosine AVLAILGNVLVCWAVWL..NSNLQNV...TNYFVVSLAAADIAVGVLAIP.FAITI---STGFCAACHGCLFIACFVLVLTQSSIFSLLAIAIDRYIAI--RIPL-RY-NGLV.TGTR.A.KGIIAICWVLSFAIG-LTPMLGW-N---beta-1 VLLIVAGNVLVIVAIAK..TPRLQTL...TNLFIMSLASADLVMGLLVVP.FGATIVVWGRW-EYGSFFCELWTSVDVLCVTASIETLCVIALDRYLAI--TSPF-RY-QSLL.TRAR.A.RGLVCTVWAISALVSFLPILMHWWRAESD beta VLAIVFGNVLVITAIAK..FERLQTV...TNYFITSLACADLVMGLAVVP.FGAAHILMKMW-TFGNFWCEFWTSIDVLCVTASIETLCVIAVDRYFAI--TSPF-KY-QSLL.TKNK.A.RVIILMVWIVSGLTSFLPIQMHWYRATHQ chemokine_r FLTGIVGNGLVILVMGY..QKKLRSM...TDKYRLHLSVADLLFVIT-LP.FWAVDAVANWY--FGNFLCKAVHVIYTVNLYSSVLILAFISLDRYLAI-VHATN-S-Q--RP.RKLL.AeKVVYVGVWIPALLLT-IPDFI--F-ANVS D2 IAVIVFGNVLVCMAVSR..EKALQTT...TNYLIVSLAVADLLVATLVMP.WVVYLEVVGEW-KFSRIHCDIFVTLDVMMCTASILNLCAISIDRYTAV--AMPML--YNTRYsSKRR.V.TVMISIVWVLSFTISC-PLLFGL-N---galanin FLLGTVGNGLVLAVLLQpgPSAWQEPgstTDLFILNLAVADLCFILCCVP.FQATIYTLDAW-LFGALVCKAVHLLIYLTMYASSFTLAAVSVDRYLAV--RHPL-R-SRALR.TPRN.A.RAAVGLVWLLAALFSA--PYLSYY----histamine ILITVAGNVVVCLAVGL..NRRLRNL...TNCFIVSLAITDLLLGLLVLP.FSAIYQLSCKW-SFGKVFCNIYTSLDVMLCTASILNLFMISLDRYCAV--MDPL-RY-PVLV.TPVR.V.AISLVLIWVISITLSFLSIHLGW-NSRNE melatonin TAVDVVGNLLVILSVLR..NRKLRNA...GNLFLVSLALADLVVAFYPYP.LILVAIFYDGW-ALGEEHCKASAFVMGLSVIGSVFNITAIAINRYCYICHSMAYHRIYR---.-RWH.T.PLHICLIWLLTV-VALLPNFFVGSL---muscarinic SLVTIIGNILVMVSIKV..NRHLQTV...NNYFLFSLACADLIIGVFSMNlYTLYT-VIGYW-PLGPVVCDLWLALDYVVSNASVMNLLIISFDRYFCV--TKPL-TY-PVKR.TTKM.A.GMMIAAAWVLSFILWA-PAILFW-----delta CAVGLLGNVLVMFGIVR..YTKMKTA...TNIYIFNLALAD-ALATSTLP.FQSAKYLMETW-PFGELLCKAVLSIDYYNMFTSIFTLTMMSVDRYIAVCHPVKALD-FR---.TPAK.A.KLINICIWVLASGVGV-PIMVMAV--TRP kappa FVVGLVGNSLVMFVIIR..YTKMKTA...TNIYIFNLALAD-ALVTTTMP.FQSTVYLMNSW-PFGDVLCKIVISIDYYNMFTSIFTLTMMSVDRYIAVCHPVKALD-FR---.TPLK.A.KIINICIWLLSSSVGISAIVLGG-TKVRE Mu-type CVVGLFGNFLVMYVIVR..YTKMKTA...TNIYIFNLALAD-ALATSTLP.FQSVNYLMGTW-PFGTILCKIVISIDYYNMFTSIFTLCTMSVDRYIAVCHPVKALD-FR---.TPRN.A.KIINVCNWILSSAIG-LPVMFMAT----orexin FVVALVGNTLVCLAVWR..NHHMRTV...TNYFIVNLSLADVLVTAICLP.ASLLVDITESW-LFGHALCKVIPYLQAVSVSVAVLTLSFIALDRWYAICHPLLF-K--S---.TARR.A.RGSILGIWAVSLAIMV--PQAAVM----rhodopsin IVLGFPINFLTLYVTVQ..HKKLRTP...LNYILLNLAVADLFMVLGGFT.STLYTSLHGYF-VFGPTGCNLEGFFATLGGEIALWSLVVLAIERYVVVCKPMSN---F--RF.GENH.A.IMGVAFTWVMALACAA-PPLAGWS----5-hydroxytryp IFCAVLGNACVVAAIAL..ERSLQNV...ANYLIGSLAVTDLMVSVLVLP.MAALYQVLNKW-TLGQVTCDLFIALDVLCCTSSILHLCAIALDRYWAI--TDPI-DYVN-KR.TPRR.A.AALISLTWLIGFLIS-IPPMLGW-R--TP somatostatin CAAGLGGNTLVIYVVLR..FAKMKTV...TNIYILNLAVAD-VLYMLGLP.FLATQNAASFW-PFGPVLCRLVMTLDGVNQFTSVFCLTVMSVDRYLAV--VHPL---SSARW.RRPRvA.KLASAAAWVLSLCMS-LPLLV--F-ADVQ TM1 TM2 TM3 adenosine NCGQP.KEGKNHSQGCGEGQVACLF-EDV-------VPMNYMVYFNF...FACVLVPLLLMLGVYLR....I...FLAAR... beta-1 EA--R.R--CYNDPKC------CD-F--VT-------NRAY-AIASS...VVSFYVPLCIMAFVYLR....V...FREAQ... beta EA--I.N--CYANETC------CD-F--FT-------NQAY-AIASS...IVSFYVPLVIMAFVYSR....V...FQEAK... chemokine_r EA--D.D--R---YI-------CDRF--YP-------NDLWVVVFQFqhiMVGLILPGIVILSCYCI....I...ISKL-... D2 NA---.---DQNE---------CIIA-----------NPAF-VVYSS...IVSFYVPFIVTLLVYIK....I...YIVLR... galanin --GTV.R--YGALEL-------CV-P--AW---EDARRRAL-DVATF...AAGYLLPVAVVSLAYGRtlrfL...WAAVG... histamine TS---.---KGNHTTS-----KCKVQ--V--------NEVY-GLVDG...LVTFYLPLLIMCITYYR....I...FKVAR... melatonin -----.---EYDPRIY-----SCT-F--IQ-----TASTQY-TAAVV...VIHFLLPIAVVSFCYLR....IwvlVLQAR... muscarinic QF--I.VGVRTVEDGE------CYIQ--FF------SNAAV-TFGTA...IAAFYLPVIIMTVLYWH....I...SRASK... delta RDGAV.VCML-QFPSP-----SWYWD-TVT------------KICVF...LFAFVVPILIITVCYGL....M...LLRLR... kappa DVDVI.ECSL---QFP-------D-D-DYS------WWDLFMKICVF...IFAFVIPVLIIIVCYTL....M...ILRLK... Mu-type ----T.K--YRQGSID------CTLT--FS--HPTWYWENLLKICVF...IFAFIMPVLIITVCYGL....M...ILRLK... orexin ECSSVlP--ELANRTR--LFSVCD--ERWA---DDLYPKIY-HSCFF...IVTYLAPLGLMAMAYFQ....I...FRKLW... rhodopsin -----.---R---YIPEGLQCSCGID--YYTLKPEVNNESF-VIYMF...VVHFTIPMIIIFFCYGQ....L...VFTVK... 5-hydroxytryp ED---.---R-SDPDA------CTISKDH----------GY-TIYST...FGAFYIPLLLMLVLYGR....I...FRAARfri somatostatin EGGTC.NASW---PEP------VGLW-----------GAVF-IIYTA...VLGFFAPLLVICLCYLL....I...VVKVR... TM5 TM4 HAA....K...SLAIIVGLFALCWLPLHI...IN....CFT...F.FC-P KAL....K...TLGIIMGVFTLCWLPFFL...AN....VVK...A.FH-KAL....K...TLGIIMGTFTLCWLPFFI...VN....IVH...V.IQ-KAL....K...TTVILILAFFACWLPYYIgisID....SFIlleI.IKQG KAT....Q...MLAIVLGVFIICWLPFFI...TH....ILN...I.HC-RATgragR...AMLAVAALYALCWGPHHA...L-....ILC...F.WY-G KAT....V...TLAAVMGAFIICWFPYFT...AF....VYR...G.LR-G RSF....L...TMFVVFVIFAICWAPLNC...IG....LAV...A.IN-P VAN....QdpvSPSLVQG---------RI...VK....PNN...N.NM-P RIT....R...MVLVVVGAFVVCWAPIHI...FV....IVW...T.LV-RIT....R...LVLVVVAVFVVCWTPIHI...FI....LVE...A.LGST RIT....R...MVLVVVAVFIVCWTPIHI..yVI....IKA...L.VTIP KTA....K...MLMVVLLVFALCYLPISV...LNvlkrVFG...M.FRQA EVT....R...MVIIMVIAFLICWVPYAS...VA....FYI...F.THQG KTV....K...TLGIIMGTFILCWLPFFI...VA....LVL...P.FCES KVT....R...MVLVVVLVFAGCWLPFFT...VN....IVN...LaVALP adenosine ...DCSHAPLW...LM...Y....LAIVLSHTNSVVNPFI-YAYRIREFRQTFRKIIRSHV-.LRQqepfkaagtsarvlaahgsdgeqvslrlnghppg beta-1 ...RE-LVPDR...LF...V....FFNWLGYANSAFNPII-Y-CRSPDFRKAFQGLLC---C.ARRaarrrhathgdrprasgclarpgpppspgaasdd beta ...DN-LIRKE...VY...I....LLNWIGYVNSGFNPLI-Y-CRSPDFRIAFQELL----C.LRRsslkaygngyssngntgeqsgyhveqekenkllc chemokine_r cefEN-TVHKW...I-...S....ITEALAFFHCCLNPIL-YAFLGAKFKTSAQHALTSVS-.-RGsslkilskgkrgghssvstesesssfhss..... D2 ...DC-NIPPV...LY...S....AFTWLGYVNSAVNPII-YTTFNIEFRKAFLKIL--HC-.---.................................. galanin ...RF-AFSPA...TYacrL....ASHCLAYANSCLNPLV-YALASRHFRARFRRLWP---C.GRRrrhrarralrrvrpassgppgcpgdarpsgrlla histamine ...DD-AINEV...LE...A....IVLWLGYANSALNPIL-YAALNRDFRTGYQQLFC---CrLANrnshktslrsnasqlsrtqsreprqqeekplklq melatonin ...QE-MAPQIpegLF...V....TSYLLAYFNSCLNAIV-YGLLNQNFRREYKRIL-----.LALwnprhciqdaskgshaeglqspappiigvqhqad muscarinic ...S-SDDGLE...--...-....-HNKIQNGKAPRDPVTENCVQGEEKESSNDSTSVSAV-.ASNmrddeitqdentvstslghskdenskqtcirigt delta ...DI-DRRDP...LV...VaalhLCIALGYANSSLNPVL-YAFLDENFKRCFRQLCR-KPC.GRPdpssfsrareatarervtactpsdgpgggaaa.. kappa ...SHSTAALS...SY...Y....FCIALGYTNSSLNPIL-YAFLDENFKRCFRDFCF---P.LKMrmerqstsrvrntvqdpaylrdidgmnkpv.... Mu-type ...ET-TFQTV...SW...H....FCIALGYTNSCLNPVL-YAFLDENFKRCFREFCIPTS-.-SNieqqnstrirqntrdhpstantvdrtnhqlenle orexin ...SDREAVYA...CF...T....FSHWLVYANSAANPII-YNFLSGKFREQFKAAF----S.CCLpglgpcgslkapsprssashkslslqsrcsiski rhodopsin ...SN--FGPI...FM...T....IPAFFAKSAAIYNPVIYI-MMNKQFRNCMLTTIC---C.GKNplgddeasatvsktetsqvapa............ 5-hydroxytryp ...SC-HMPTL...LG...A....IINWLGYSNSLLNPVI-YAYFNKDFQNAFKKIIKCKF-.CRQ.................................. somatostatin ...QE-PASAG...LY...F....FVVILSYANSCANPVL-YGFLSDNFRQSFQKVL----C.LRKgsgakdadateprpdrirqqqeatrprtaaangl TM7 Fig 19.Sequence aligment of many class A receptors based on hmm model TM6 3. GPCRs Nomenclature Fig 20. Scheme of structure of the class A A2A B1AR Rhodopsin B2AR ----------------------------------IMGSSVY-----ITVELAIAVLAILGNVLVCWAVWLNSNLQNVTNYFVVSLAAADIAVGVLAIPFAITIS---TGFCAACHGCLFI -----------------------------------------WEAGMSLLMALVVLLIVAGNVLVIAAIGSTQRLQTLTNLFITSLACADLVVGLLVVPFGATLVVRGTWL-WGSFLCELW MNGTEGPNFYVPFSNKTGVVRSPFEAPQYYLAEPW-QFSML-----AAYMFLLIMLGFPINFLTLYVTVQHKKLRTPLNYILLNLAVADLFMVFGGFTTTLYTSLHGYFV-FGPTGCNLE --------------------------------DEV-WVVGM-----GIVMSLIVLAIVFGNVLVITAIAKFERLQTVTNYFITSLACADLVMGLAVVPFGAAHILMKMWT-FGNFWCEFW ClassA_stamp_VMDA2A ClassA_stamp_VMDAvi ClassA_stamp_VMDBov ClassA_stamp_VMDHum ----------------------------------CCHHHHH-----HHHHHHHHHHHHHHHHHHHHHHHHCGGGCCHHHHHHHHHHHHHHHHHHHHHHHHHHHH---CCCEEEHHHHHHH -----------------------------------------HHHHHHHHHHHHHHHHHHHHHHHHHHHHTTGGGCCHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHCCCC -CCHHHHHHH CCCEETTTTEECCCTTTTCCCTTTTTCCGGGCCHH-HHHHH-----HHHHHHHHHHHHHHHHHHHHHHHHTTTTCCHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHCCTT-TTHHHHHHH --------------------------------CHH-HHHHH-----HHHHHHHHHHHHHHHHHHHHHHHHCGGGTTHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHCCTT-TTHHHHHHH ClassA_stamp_VMDA2A ClassA_stamp_VMDAvi ClassA_stamp_VMDBov ClassA_stamp_VMDHum CFVLVLTQSSIFSLLAIAIDRYIAIR--IPLR-YN-GLVTGTRAKGIIAICWVLSFAIGL-TPMLGW-N-N-----CGQSQGCGEGQVA---C------LF-E-DVV------PMNYMV TSLDVLCVTASIETLCVIAIDRYLAIT--SPFR-YQ-SLMTRARAKVIICTVWAISALVSFLPIMMHWWR-DEDPQAL---KCY----QDPGCC------DF-V---T------NRAYAGFFATLGGEIALWSLVVLAIERYVVVCKPMSNF--R---FGENHAIMGVAFTWVMALACAA-PPLVGWSRY---------------------IPEGMQCSCGIDYY-TPHEETNNESFVTSIDVLCVTASIETLCVIAVDRYFAIT--SPF-KYQSL-LTKNKARVIILMVWIVSGLTSFLPIQMHWYR-ATHQEAI---NCY----AEETCC------DF-F---T------NQAYA- ClassA_stamp_VMDA2A ClassA_stamp_VMDAvi ClassA_stamp_VMDBov ClassA_stamp_VMDHum HHHHHHHHHHHHHHHHHHHHHHHHTTT--TTTT-TT-TTTTHHHHHHHHHHHHHHHHHHHH-GGGGTT-T-T-----TTCCTTTTTTEEE---T------TG-G-GCC------CHHHHH HHHHHHHHHHHHHHHHHHHHHHHHHTT--THHH-HH-HHCCHHHHHHHHHHHHHHHHHHHHHHHHHTTTB-CCCHHHH---HHH----HTTTTT------CC-C---B------CHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHTTCTTTT--C---CCHHHHHHHHHHHHHHHHHHHH-HHHHTTTTE---------------------EEETTTCEEEETTT-TCTTTTTHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHHH--TTT-TTTCC-CCHHHHHHHHHHHHHHHHHHHHHHHHHTTTB-CCCHHHH---HHH----HTTTTC------TT-T---B------CHHHH- ClassA_stamp_VMDA2A ClassA_stamp_VMDAvi ClassA_stamp_VMDBov ClassA_stamp_VMDHum YFNFFACVLVPLLLMLGVYLRIFLAARR--QLNIFEMLRIDEGLRLKIYKDTEGYYTIGIGHLLTKSPSLNAAKSELDKAIGRNTNGVITKDEAEKLFNQDVDAAVRGILRNAKLKPVYD IASSIISFYIPLLIMIFVALRVYREAKEQI-----------------------------------------------------------------------------------------IYMFVVHFIIPLIVIFFCYGQLVFTVK--------------------------------------------------------------------------------------------IASSIVSFYVPLVIMVFVYSRVFQEAKRQ-LN----I----------------------------------------------------------------------------------- ClassA_stamp_VMDA2A ClassA_stamp_VMDAvi ClassA_stamp_VMDBov ClassA_stamp_VMDHum HHHHHHHHHHHHHHHHHHHHHHHHHHCC--CCCHHHHHHHHHCCEEEEEETTTTCEEEETTEEEECCCCHHHHHHHHHHHHCCCTTTBCCHHHHHHHHHHHHHHHHHHHHHCCHHHHHHH HHHHHHHHHHHHHHHHHHHHHHHHHHHHCC-----------------------------------------------------------------------------------------HHHHHHHCHHHHHHHHHHHHHHTTTTT--------------------------------------------------------------------------------------------HHHHHHHHHHHHHHHHHHHHHHHHHHHHH-CC----H----------------------------------------------------------------------------------- ClassA_stamp_VMDA2A ClassA_stamp_VMDAvi ClassA_stamp_VMDBov ClassA_stamp_VMDHum SLDAVRRAALINMVFQMGETGVAGFTNSLRMLQQKRW--------------------------DEAAVNLA---------K--SRWYN-------QTPNRAKRVITTFR---TG----------------------------------------------------------------------------------------------------------------- ----------------------------------------------------------------------------------------------------------------------- ------------------------------------------------FEMLRIDEGLRLKIYKDTEGYYTIGIG--HL--LTKSPSLNAAKSELDKAIGRNTNGVIT-KDEAEKLFNQDVDAAVRGILRN ClassA_stamp_VMDA2A ClassA_stamp_VMDAvi ClassA_stamp_VMDBov ClassA_stamp_VMDHum HCCHHHHHHHHHHHHHHCHHHHHTTHHHHHHHHHCCH--------------------------HHHHHHHH---------C--CHHHH-------HCHHHHHHHHHHHH---HC----------------------------------------------------------------------------------------------------------------- ----------------------------------------------------------------------------------------------------------------------- ------------------------------------------------HHHHHHHHCCEEEEEETTTTCEEEETT--EE--EECCCCHHHHHHHHHHHHCCCTTTBCC-HHHHHHHHHHHHHHHHHHHHHT ClassA_stamp_VMDA2A ClassA_stamp_VMDAvi ClassA_stamp_VMDBov ClassA_stamp_VMDHum --------------TW---DAYR-----------------------------------------------------------S------TL-QKEVHAAKSLAIIVGLFALCWLPLHIIN --------------------------------------------------------------------------------------------- REHKALKTLGIIMGVFTLCWLPFFLVN --------------------------------------------------------------------------------E--AAASATTQ-KAEKEVTRMVIIMVIAFLICWLPYAGVA AKLKPVYDSLDAVRRAALIN---MVFQMGETGVAGFTNSLRMLQQKRWDEAAVNLAKSRWYNQTPNRAKRVITTFRTGTWDAYKF------CLKEHKALKTLGIIMGTFTLCWLPFFIVN ClassA_stamp_VMDA2A ClassA_stamp_VMDAvi ClassA_stamp_VMDBov ClassA_stamp_VMDHum --------------CH---HHHH-----------------------------------------------------------H------HH-HHHCHHHHHHHHHHHHHHHHHHHHHHHH --------------------------------------------------------------------------------------------- HHHHHHHHHHHHHHHHHHHHHHHHHHH --------------------------------------------------------------------------------T--TCCCCCCH-HHHHHHHHHHHHHHHHHHHHHHHHHHHH TTHHHHHHHCCHHHHHHHHH---HHHHHHHHHHHHCHHHHHHHHHCCHHHHHHHHHCCHHHHHCHHHHHHHHHHHHHCCCGGGTT------TCHHHHHHHHHHHHHHHHHHHHHHHHHHH ClassA_stamp_VMDA2A ClassA_stamp_VMDAvi ClassA_stamp_VMDBov ClassA_stamp_VMDHum CFTFFCPDCSHAPLWLMYLAIVLSHTNSVVNPFIYAYRIREFRQTFRKIIRSHVLR --Q---------------IVNVFNRDL--VPDWLFVAFNWLGYANSAMNPIIYC-RSPDFRKAFKRLLA-----------------------FYIFTHQGSD-FGPIFMTIPAFFAKTSAVYNPVIYIMMNKQFRNCMVTTLCC----GKNPSTTVSKTETSQVAPA IVHVIQDNL--IRKEVYILLNWIGYVNSGFNPLIYC-RSPDFRIAFQELLC-------L---------------- ClassA_stamp_VMDA2A ClassA_stamp_VMDAvi ClassA_stamp_VMDBov ClassA_stamp_VMDHum HHHHHTTTTCCCCHHHHHHHHHHHHHHCHHHHHHHHHHCHHHHHHHHHHHHHHHCC --C---------------HHHHHTTTT--CCHHHHHHHHHHHHHHHHHHHHHHH-HCHHHHHHHHHHHC-----------------------HHHHHTTTTC-CCHHHHHHHHHHHGGGGHHHHHHHHHHCHHHHHHHHHHHHT----TTCCCCCCTTTTTTCCCCC HHHHHTTTT--TTHHHHHHHHHHHHHHHCHHHHHHH-CCHHHHHHHHHHHC-------C---------------- TM1 TM3 TM2 TM4 TM5 Lysozyme Lysozyme Lysozyme TM7 Fig 21. Structural alignment of class A receptors TM6 3. GPCRs Structural Conservation SCORE 5.57 RMSD 1.92 Fig22. Superimposition of β2AR, β1AR, A2AR and Rhodopsin Fig23. Superimposition of β2AR, β1AR, A2AR and Rhodopsin 3. GPCRs Structural Conservation Fig24. Superimposition of β2AR, β1AR, A2AR and Rhodopsin 3. GPCRs β2 Adrenergic Receptor >beta 2 adrenergic receptor [Homo sapiens] MGQPGNGSAFLLAPNRSHAPDHDVTQQRDEVWVVGMGIVMSLIVLAIVFGNVLVITAIAKF ERLQTVTNYFITSLACADLVMGLAVVPFGAAHILMKMWTFGNFWCEFWTSIDVLCVTASIET LCVIAVDRYFAITSPFKYQSLLTKNKARVIILMVWIVSGLTSFLPIQMHWYRATHQEAINCYA NETCCDFFTNQAYAIASSIVSFYVPLVIMAFVYSRVFQEAKRQLQKIDKSEGRFHVQNLSQVE QDGRTGHGLRRSSKFCLKEHKALKTLGIIMGTFTLCWLPFFIVNIVHVIQDNLIRKEVYILLNW IGYVNSGFNPLIYCRSPDFRIAFQELLCLRRSSLKAYGNGYSSNGNTGEQSGYHVEQEKENKL LCEDLPGTEDFVGHQGTVPSDNIDSQGRNCSTNDSLL Fig25. β2 Adrenergic Receptor sequence β2AR G proteins Adenilate cyclase AMPc PKA Smooth muscle relaxation, blood vessels dilatation, striated muscle contraction, bronchiole dilation... • The first non-rhodopsin GPCR cloned • One of the most extensively studied members of this large receptor family. 3. GPCRs β2 Adrenergic Receptor MEMSAT software to predict alpha helix transmembrane regions based on Dr. Jones predictive algorithm described in 1994 Fig26 Hydropathy plot of β2 Adrenergic Receptor sequence Fig27. Hydropathy plot of β2 Adrenergic Receptor sequence 3. GPCRs β2 Adrenergic Receptor ECL2 Extracellular II VI IV VII III I carazolol V VIII T4-lysozyme Fig28. β2 Adrenergic Receptor Intracellular 3. GPCRs β2 Adrenergic Receptor TM1 TM2 TM3 TM4 TM5 TM6 TM7 Fig29. Sequence and secondary structure of β2 Adrenergic Receptor sequence obtained from PDB 3. GPCRs β2 Adrenergic Receptor ECL2 Extracellular II VI IV VII III I carazolol V VIII T4-lysozyme Fig30. β2 Adrenergic Receptor Intracellular 3. GPCRs ECL2 (Extracellular Loop 2) ECL2 Cys 190 Cys 184 Cys 191 Cys 106 Phe 193 Carazolol Fig31. Extracellular Loop 2 of the β2 Adrenergic Receptor 3. GPCRs ECL2 (Extracellular Loop 2) ECL2 Cys 190 Rhodopsin Cys 184 Cys 191 Cys 106 ECL2 Phe 193 Cys 187 Cys 110 Carazolol Fig32. Extracellular Loop 2 of the β2 Adrenergic Receptor β2-Adrenergic Receptor Retinal Fig33. Extracellular Loop 2 of Rhodopsin 3. GPCRs ECL2 (Extracellular Loop 2) ECL2 Cys 159 Cys 71 Cys 146 ECL1 Cys 166 Cys 74 Cys 77 Fig34. Extracellular Loop 2 of β2-Adrenergic Receptor, Rhodopsin and A2- Adenosin Receptor with the ligands carazolol and retinal Fig36. Extracellular Loop 2 of β2-Adrenergic Fig35. A2- Adenosin Receptor Receptor,with Rhodopsin its three disulfur and A2bonds. Adrenergic Receptor with their conserved disulfur bonds. 3. GPCRs β2 Adrenergic Receptor ECL2 Extracellular II VI IV VII III I carazolol V VIII T4-lysozyme Fig37. β2 Adrenergic Receptor Intracellular 3. GPCRs Ligand binding site Fig38. Extracellular view of the ligand binding site of the β2 Adrenergic Receptor with its ligand carazolol 3. GPCRs Ligand binding site Carazolol Ser203 Ser203 Asp113Asn312 Ser203 Ser203 Asp113 Carazolol Asn312 Fig39. Interaction between β2-Adrenergic Receptor and carazolol Fig40. Interaction between β2-Adrenergic Receptor and carazolol 3. GPCRs Ligand binding site Trp109 Trp109 Tyr199 Phe193 Val114 Trp286 Phe193 Val114 Phe289 Phe290 Phe289 Tyr199 Phe290 Trp286 Fig42. Interaction Fig41. Interaction between between β2-Adrenergic β2-Adrenergic Receptor Receptor and carazolol and carazolol 3. GPCRs Ligand binding site Fig43. Interaction between β2-Adrenergic Receptor and carazolol compared to the interaction between Rhodopsin and retinal 3. GPCRs Ligand binding site Fig44. Interaction between β2-Adrenergic Receptor and carazolol compared to the interaction between Rhodopsin and retinal 3. GPCRs Ligand binding site Phe5.47 Tyr6.51 Tyr6.51 Trp6.48 Phe5.47 Trp6.48 Fig45. Superimposition of the ligand binding site of Rhodopsin and β2-Adrenergic Receptor Fig46. Superimposition of the ligand binding site of Rhodopsin and β2-Adrenergic Receptor 3. GPCRs Ligand binding site Rhodopsin β2-Adrenergic Receptor Trp6.48 Trp6.48 Fig47. Interaction between Rhodopsin and retinal through Trp6.48 Fig48. Interaction between β2-Adrenergic Receptor and carazolol and Trp6.48 “Toogle Switch” 3. GPCRs β2 Adrenergic Receptor ECL2 Extracellular II VI IV VII III I carazolol V VIII T4-lysozyme Fig49. β2 Adrenergic Receptor Intracellular 3. GPCRs Ionic Lock Rhodopsin β2-Adrenergic Receptor Tyr3.51 Tyr3.51 Tyr3.51 Asp3.49 Glu/Asp3.49 Arg3.50 Arg3.50 Arg3.50 Glu3.49 Glu6.30 Glu6.30 Fig50. Ionic lock of Rhodopsin Glu6.30 Fig51. Ionic lock of β2-Adrenergic Receptor Conserved E/D(E)RY motif Fig52. Superimposition of the Ionic lock of Rhodopsin and β2-Adrenergic Receptor 3. GPCRs Activation ECL2 ECL2 II VI IV VII Extracellular Extracellular III II IV I carazolol V BI-167107 V VIII I VII VIII III VI T4-lysozyme Fig53. β2 Adrenergic Receptor Nanobody (Nb80) Intracellular Intracellular Fig54. Nanobody stabilized-β2 Adrenergic Receptor 3. GPCRs Activation Fig55. Conserved prolines in β2 Adrenergic Receptor 3. GPCRs Activation I ECL2 II VII III IV I II IV V ICL2 VI III VII V VI Fig56. Superimposition of β2 Adrenergic Receptor in its activated and inactivated states. Fig57. Superimposition of β2 Adrenergic Receptor in its activated and inactivated states. 3. GPCRs Activation Tyr199 Phe193 Phe193 Trp109 Tyr199 Val114 Val114 Phe290 Phe290 Trp286 Phe289 Ile309 Fig58. Interaction between β2-Adrenergic Receptor and BI-167107 Trp286 Phe289 Fig59. Interaction between β2-Adrenergic Receptor and carazolol 3. GPCRs Activation Carazolol BI-167107 Ser203 Ser203 Asp113 Asp113 BI-167107 Ser203 Asn312 Asn312 Asn312 Ser207 Fig60. Interaction between β2-Adrenergic Receptor and BI-167107 Fig61. Interaction between β2-Adrenergic Receptor and carazolol Fig62. Interaction between β2-Adrenergic Receptor and BI-167107 3. GPCRs Activation Ser2075.46 Ser203 BI-167107 carazolol Ser207 Ser203 Pro2115.50 Ser207 Ile1213.40 Ile121 Asn318 Asn3187.45 Pro2115.50 Ile1213.40 Pro211 Phe2826.44 Asn3187.45 Phe282 Fig64. Adrenergic Receptor in its activated state. Fig63. β2 Adrenergic Receptor in its inactivated state. Phe2826.44 Phe2826.44 Fig65. Superimposition of β2 Adrenergic Receptor in its activated and inactivated states. 3. GPCRs Activation Trp6.48 Fig66. Superimposition of β2 Adrenergic Receptor in its activated and inactivated states. 3. GPCRs Activation Glu268 Arg131 Fig67. Superimposition of β2 Adrenergic Receptor in its activated and inactivated states. 3. GPCRs Activation Glu268 Arg131 Fig68. β2 Adrenergic Receptor coupled to G-protein 4.Conclusions G-protein-coupled receptors represent the largest class of drug targets, and its structure determination is very important to design new specific drugs. Although the crystallization methods have improved incredibly, we need to crystallize new states of structures that have already been crystallized to understand better GPCR dynamics such as the activation process. In addition, the crystallization of new structures is needed to amplify the knowledge of these receptors and to design new drugs. 5. Bibliography Costanzi S. On the Applicability of GPCR Homology Models to Computer-Aided Drug Discovery: A Comparison between In Silico and Crystal Structures of the β2- Adrenergic receptor. J. Med.Chem. 2008; 51: 2907-2914. Cherezov V, Rosenbaum D.M, Hanson M.A, Rasmussen S.G.F, Thian F.S, Kobilka T.S., Choi H, Kuhn P, Weis W.I, Kobilka B.K, Stevents R.C. High – Resolution Crystal Structure of an Engineered Human β2Adrenergic G Protein – Coupled Receptor. Deupi X, Kobilka B.K. Energy landscapes as a tool to integrate GPCR structure, dynamics, and function. Physiology (Bethesda). 2010: 25; 293–303 Deupi X, Standfuss J. Structural insights into agonist-induced activation of G-protein-coupled receptors. Current Opinion in Structural Biology. 2011; 21: 541 – 551. Dror R. O, Arlow D. H, Maragakis P, Mildorf T. J, Pan A. C, Xu H, Borhani D. W, Shaw D. E. Activation mechanism of the β2 - Adrenergic receptor. PNAS. 2011; 108: 18684 – 18689. Fairman J.W, Noinaj N, Buchanan S.K. The structural biology of β- barrel membrane proteins: a summary of recent report. Current Opinion Structural Biology. 2011; 21: 523 – 531. Goetz A, Lanig, Gmeiner P, Clark T. Molecular Dynamics Simulations of the Effect of the G-Protein and Diffusible Ligands β2-Adrenergic Receptors. J. Mol.Biol. 2011; 414: 611 – 623. González A, Perez-Acle T, Pardo L, Deupi X. Molecular Basis of Ligand Dissociation in β-Adrenergic Receptor. Plos ONE. 2011; 6. 5. Bibliography Jones D.T., Taylor W.R., Thornton J.M. A model recognition Approach to the prediction of All – Helical Membrane Protein Structure and Toxicology. Biochemistry. 1994; 33 (10): 3038-3049. Kahsai A. W, Xiao K, Rajagopal S, Ahn S, Shukla A, Sun J, Oas T. G, Lefkowitz R. J. Multiple ligand – specific conformations of the β2- adrenergic receptor. Nature Chemical Biology. 2011; 7: 692 – 700. Katritch V, Abagyan R. GPCR agonist binding revealed by modeling and crystallography. Trends in Pharmacological Science. 2011; 31 (11): 637 – 643. Kobilka B.K. Structural insights into andrenergic receptor function and pharmacology. Trends in Pharmacological Science. 2011; 21 (4): 213-218. Kolb P, et al. Structure-based discovery of β2- Adrenergic receptor ligands. Proc. Natl. Acad. Sci. U.S.A. 2009; 106: 6843–6848López Muñoz L. Homology modeling and structural analysis of the antipsychotic drugs receptorome. [Doctoral Thesis]. Universitat Pompeu Fabra; 2010. Massotte D, Kieffler B. L. Structure – Function Relationships in G Proteins – Coupled Receptors Handbook. In: Devi L. A, editor. The G Protein – Coupled Receptors. New Yersey: Humana Press; 2005. 339. Palczewski K, et al. Crystal structure of rhodopsin: a G protein-Coupled receptor. Science. 2000; 289: 739– 745. 5. Bibliography Rasmussen S.G, et al. Crystal structure of the human β2 -Adrenergic G-protein-coupled receptor. Nature. 2007; 450: 383–387. Rasmussen S.G, DeVree B.T, Zou Y., Kruse A.C, Chung K.Y, Kobilka T.S. Crystal structure of the β2 Adrenergic Receptor-Gs protein complex. Nature. 2011; 477: 549-555. Rasmussen S.G, et al. Structure of a nanobody-stabilized active state of the β2 - adrenoceptor. Nature. 2011; 469: 175–180. Rosenbaum D.M,et al. GPCR engineering yields high-resolution structural insights into β2 -adrenergic receptor function. Science. 2007; 318: 1266–1273 Rosenbaum D. M, Rasmussen S. G, Kobilka B.K. The β2- Adrenergic Receptor as a Model for G-ProteinCoupled Receptor Structure and Activation by Diffusible Hormones. Handbook of Cell Signaling. 2010; Chapter 25: 163-168. Rosenbaum D.M, et al. Structure and function of an irreversible agonist-β2 adrenoceptor complex. Nature. 2011; 469: 236–240. Sabio M, et al. Use of the X-ray structure of the β2- Adrenergic receptor for drug discovery. Part 2: identification of active compounds. Bioorg. Med. Chem. Lett. 2008; 18: 5391–5395. Sansuk K, et al. A structural insight into the reorientation of transmembrane domains 3 and 5 during family A GPCR activation. Mol. Pharmacol. 2011; 79: 262–269 5. Bibliography Schulz G. β- barrel membrane proteins. Current Opinion in Structural Biology. 2000; 10: 443 – 447. Topiol S, Sabio M. X-ray structure breakthroghs in the GPCR transmembrane region. Biochemical Pharmacology. 2009; 79: 11 – 20. Warne T, et al. Structure of a β1- Adrenergic G-protein-coupled receptor. Nature. 2008; 454: 486–491. Weis W. I, Kobilka B. K. Structural insights into G-protein-coupled receptor activation. Current Opinion Structural Biology. 2008; 18: 734 – 740. Wimley W.C. The versatile β- barrel membrane protein. Current Opinion in Structural Biology. 2003; 13: 404 – 411. Worth C. L., Kleinau G, Krause G. Comparative Sequence and Structural Analysen of G-protein-coupled Receptor Crystal Structures and Implications for Molecular Models. Plos One. 2009; 9. THANKS FOR YOUR ATTENTION!!!! QUESTIONS?





![Shark Electrosense: physiology and circuit model []](http://s2.studylib.net/store/data/005306781_1-34d5e86294a52e9275a69716495e2e51-300x300.png)