A2_Unit5_Nuclear_13_Binding_Energy
advertisement

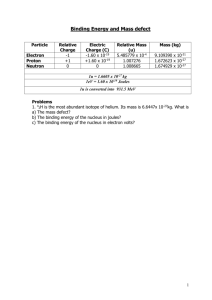
Binding Energy Nuclear Physics Lesson 13 Learning Objectives To define binding energy. To define mass defect. To know which are the most stable nuclei. Explain why energy is released in nuclear fission and nuclear fusion. The Atomic Mass Unit The atomic mass unit (u) is far more convenient to use with nuclear masses. It uses carbon-12 as a reference and is defined as: Exactly 1/12th the mass of a carbon 12 atom. 1 atomic mass unit (u) = 1.661 × 10-27 kg. The table shows particle masses in atomic mass units. Note that the numbers are expressed to a large number of significant figures as the changes are quite subtle. Particle Mass (u) Electron Neutron Proton Hydrogen atom (1p+ + 1e-) 0.000549 1.008665 1.007276 1.007825 Helium atom (2p+ + 2n + 2e-) 4.002603 α particle (2p+ + 2n) 4.001505 Be Careful! Remember to distinguish between the atomic mass and the nuclear mass. The atomic mass is the mass of an atom complete with its electrons. The nuclear mass is the mass of the nucleus alone. To get the nuclear mass we need to take away the mass of the electrons. Binding Energy If you want to remove a nucleon from the nucleus of an atom, then you have to do work to overcome the strong nuclear force. Definition from specification book:- The binding energy of a nucleus is the work that must be done to separate a nucleus into its constituent neutrons and protons. The removed nucleons gain potential energy. Binding Energy II If we run that process in reverse, then the binding energy can also be defined as the energy released when a nucleus is assembled from its constituent nucleons. This means that the mass of the nucleus is less than the mass of the separate nucleons because energy has been released… Mass Defect Definition from the specification book:- The mass defect Δm of a nucleus is defined as the difference between the mass of the separated nucleons and the mass of the nucleus. Calculating Mass Defect For a nucleus of an isotope ZAX, composed of Z protons and (A-Z) neutrons with mass MNUC, the mass defect, Δm, is given by:- m ZmP ( A Z )mN M NUC Question 1 What is the mass defect of a helium atom? Answer 1 What is the mass defect of a helium atom? Particle Mass (u) Number Total (u) Proton Neutron 1.007276 1.008665 2 2 2.014552 2.017330 Electron 0.000549 2 0.001098 Total 4.032980 The atomic mass of a helium atom is 4.002603 u Therefore mass defect, Δm=0.030377 u Calculating Binding Energy The mass defect Δm exists because energy is released when the constituent nucleons bind together to form a nucleus. The energy released is equal to the binding energy of the nucleus:- binding energy of a nucleus mc 2 Note that Δm must be in kg to get energy in J. Question 2 What is the binding energy of the helium atom whose mass defect is 0.030377 u? Express your answer in MeV. m = 0.030377 u × 1.661 × 10-27 kg = 5.046 × 10-27 kg E = mc2 = 5.046 × 10-27 kg × (3 × 108)2 = 4.541 × 1012 J E = 4.541 × 10-12 J = 28.38 × 106 eV= 28.38 MeV Note: 1 u = 931.3 MeV Binding Energy Per Nucleon If we know the binding energy in a nucleus, and the number of nucleons, we can work out the binding energy per nucleon, which is the work done needed to remove each nucleon. The higher the binding energy per nucleon, the more stable is the nucleus. For helium (4He) the binding energy per nucleon is: Binding energy per nucleon = 28.38 MeV/4 = 7.1 MeV Question 3 What is the mass defect in atomic mass units (u) and in kilograms for the lithium nucleus which has 7 nucleons, and a proton number of 3? What is the binding energy in J and eV? What is the binding energy per nucleon in eV? The nuclear mass = 7.014353 u. Answer 3 Add them together to get 7.056488 u Now take away the nuclear mass from the number above to get the mass deficit. 7.056488 u - 7.014353 u = 0.042135 u Now convert to kilograms: 1 u = 1.661 ´ 10-27 kg 0.042135 u × 1.661 ´ 10-27 kg = 6.9986235 × 10-29 kg Now use E = mc2 to work out the binding energy: E = 6.9986235 × 10-29 kg × (3 × 108 m/s)2 = 6.3 × 10-12 J In electron volts, this is 6.3 × 10-12 J ÷ 1.6 × 10-19 eV/J = 3.9 × 107 eV = 39 MeV. There are 7 nucleons so the binding energy per nucleon = 3.9 × 107 eV ÷ 7 = 5.6 × 106 eV Question 4 What is the mass defect in atomic mass units (u) and in kilograms for the copper nucleus which has 63 nucleons, and a proton number of 29? What is the binding energy in J and eV? What is the binding energy per nucleon in eV? The nuclear mass = 62.91367 u. Answer 4 Number of protons = 29; number of neutrons = 63 – 29 = 34 Mass of protons = 29 ´ 1.007276 = 29.211004 u Mass of neutrons = 34 ´ 1.008665 = 34.29461 u Total mass = 29.211004 u + 34.29461 u = 63.505614 u Mass defect = 63.505614 u – 62.91367 u = 0.591944 u Mass defect in kg = 0.591944 ´ 1.661 ´ 10-27 = 9.83218 ´ 10-28 kg Binding energy = mc2 = 9.83218 ´ 10-28 kg ´ (3 ´ 108 m/s)2 = 8.85 ´ 10-11 J Binding energy in eV = 8.85 ´ 10-11 J ¸ 1.6 ´ 10-19 J/eV = 5.53 ´ 108 eV Binding energy per nucleon = 5.53 ´ 108 eV ¸ 63 = 8.78 ´ 106 eV Binding Energy per Nucleon We can plot a graph of binding energy per nucleon against nucleon number. From this graph we can see that The maximum value is about binding energy of 8.7 MeV per nucleon and iron is the most stable nuclide. Helium has a particularly high value of binding energy per nucleon, much higher than the light isotopes of hydrogen. There is a trend for nuclides of nucleon numbers in multiples of 4 to be particularly stable (i.e. have a high binding energy). The largest nuclides tend to be less stable, with slightly lower binding energies per nucleon. The Most Stable Nucleus Iron has the highest binding energy per nucleon so is the most stable nucleus. If we look at large nuclei (greater than iron), we find that the further to the right (greater nucleon number) the less stable the nuclei. This is because the binding energy per nucleon is getting less. The explanation for this observation lies in that the strong nuclear force that binds the nucleus together has a very limited range, and there is a limit to the number of nucleons that can be crammed into a particular space. Nuclear Fission A large unstable nucleus splits into two fragments which are more stable than the original nucleus. The binding energy per nucleon increases in this process and energy is released. The change in binding energy per nucleon is about 0.5 MeV in a fission reaction. Nuclear Fusion Small nuclei fuse together to form a larger nucleus. The product nucleus has a higher binding per nucleon as long as A is no greater than ~50. The change in binding energy per nucleon can be more than 10 times greater in a fusion reaction than a fission reaction. Summary Atomic Mass Unit: 1/12th the mass of a carbon atom Mass defect: Difference between the mass of nucleons separately and together within a nucleus. Difference between the two sides of a nuclear interaction equation. Energy worked out by E = mc2. Binding Energy: Energy equivalent of the mass defect in a nucleus. Binding energy per nucleon increases in more stable nuclei. Fission Splitting of a nucleus. Rarely spontaneous. Occurs after the nucleus has been tickled with a neutron Fusion Joining together of two light nuclei to make a heavier nucleus.