Nuclear Reactions Conservation of Energy Q of the reaction Binding
advertisement

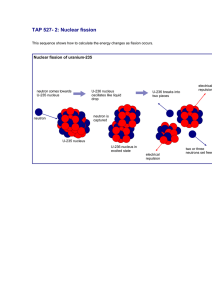
Nuclear Reactions When two nucleon combine to form two or more nuclear particles +→ + Laws 1. Conservation of nucleons 2. Conservation of charge 3. Conservation of energy 4. Conservation of momentum Q of the reaction • Recall: 1u = 1.66 x 10-24g = 932 MeV • So ( = + − ! + " 932,• If Q>0 : Net increase in KE reaction is exothermic • If Q<0 : Net decrease in KE reaction is endothermic • Ex… Find the Q of the reaction • Solution.. M(N14) = 14.0067 M(n) = 1.0086 15.0153 u M(C14)=14.0032 M(H1) = 1.0079 15.0111 u • Q = (+0.0042)932MeV = +3.9 MeV exothermic Conservation of Energy + + + = = ! +! + " + " • Rearranging (! +" ) − + = [ + − ! + " ] • Define the Q of the reaction as the change in the rest mass energy in MeV ( = [ + − ! + " ] • Ex… Complete the following reaction /0. + /1 → ? + /3 1. Z (atomic #) for Nitrogen = 7 2. Z for n = 0 3. Z for H = 1 4. Z for ? = 7+0=?+1 => ? = 6=C 5. A for N = 14 6. A for n = 1 7.A for H = 1 8. A for ? = 14+1=?+1 => ? =14 /0. + /1 → /0 + /3 Binding Energy and Mass Defect • The mass of all nuclei are somewhat smaller than the sum of the masses of the neutrons and protons contained in them. This mass defect (∆) is • ∆ = 56 + .7 − 8 mass of protons mass of neutrons mass of nucleus • Mass is converted to KE 1 BE and mass defect Binding Energy per Nucleon (BE/A) • When ∆ is expressed in energy units, it is equal to the energy that is necessary to break the nucleus into its component nucleons. This amount of energy is called the Binding Energy (BE) since it represents the energy necessary to hold the nucleus together. • Binding Energy (BE) = Mass Defect (∆) expressed in energy units. • Note ∆ = 5 6 + 9: + .7 − (8 + 59: ) mass of mass of neutral /3 neutral atom Thus we can use the tabulated masses of neutral atoms to compute ∆, Q and BE. • The total BE of a nuclei is an increasing function of the atomic number A. However, it does not increase at a constant rate. • ;<⁄8 = ∆⁄8 is the average BE per nucleon. It decreases for large A. BE/A Q in terms of BE • BE/A is a measure of stability of the nucleus. A large BE/A means strong bonds and stability. • Because of the shape of the BE/A curve it is possible for a heavy nuclide to split (fission) into two lighter fragments that each have a larger BE/A and release energy in the process. • Recall + → + • where ∆ = 5 ( /3) + . 7 − so = 5 ( /3) + . 7 − ∆() and = 5 ( /3) + . 7 − ∆() and ! = 5! ( /3) + .! 7 − ∆() and " = 5" ( /3) + ." 7 − ∆() Since ( = [ + − ! + " ] Thus ( = > + > BE and ∆ ( = > + > − [> + > ] • If the total BE of the products (c + d) is greater than BE of the reactants (a +b), then Q is positive and the reaction is exothermic. • Therefore, whenever a more stable configuration is achieved by combining less stable nucleons energy is released! − [> + > ] Fission • In the region of large A, a more stable configuration is formed when the heavy nucleus splits. • Consider BE/A(U-238) = 7.5 MeV and BE/A(?-119) = 8.4 MeV @A? → //B? + //B? so if Then there will be a gain of ~0.9 MeV per nucleon for a total of 238 x 0.9MeV = 214 MeV per fission. This is the source of energy in nuclear reactors. 2 Chapter 2 Summary Fusion @ / • Consider 2 3 → 3 + 3 • • • • deuterium tritium Two deuterons have BE(H-2)= 2 x 2.23 MeV Form @3 with BE(H-3) = 8.48 MeV and BE(H-1) = 0 (since only one nucleon) Q = 8.48 – 2 x 2.23 = +4.02 MeV which appears as the KE of @3 and H. Two light less stable nuclei produce a heavier but more stable nucleus. • • • • • • • • • Fundamental Particles Nuclides and isotopes Atom percent and weight percent Atom density Mass and energy Radioactive decay Half life Nuclear reactions Mass defect, Binding energy , & Q of reaction 3