Problem-Solving Strategy

Problem-Solving Strategy
Nuclear Properties
IDENTIFY the relevant concepts: The key properties of any nucleus include the mass, radius, binding energy, mass defect, binding energy per nucleon, and angular momentum.
SET UP the problem: Once you have identified your target variables, collect the equations you need to solve the problem. A relatively small number of equations from this section and Section 43.1 are all you need, but make sure that you understand their meanings.
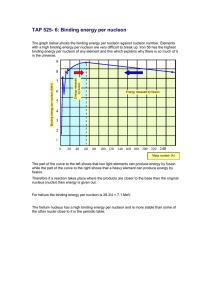
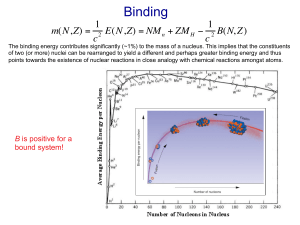
EXECUTE the solution: Solve the equations for the target variables. When doing energy calculations involving the binding energy and binding energy per nucleon, note that mass tables almost always list the masses of neutral atoms, including their full complement of electrons. To compensate for this, use the mass of a atom in Eq. (43.10) rather than the mass of a bare proton. The binding energies of the electrons in the neutral atoms are much smaller and tend to cancel in the subtraction in Eq. (43.10), so we won’t worry about them. As Eq. (43.10) shows, binding energy calculations often involve subtracting two nearly equal quantities. To get enough precision in the difference, you often have to carry seven, eight, or nine significant figures, if that many are available. If not, you may have to be content with an approximate result.
EVALUATE your answer: Familiarity with some typical numerical magnitudes is very useful when checking your results. Many of these magnitudes are quite different in nuclear physics than in atomic physics. Protons and neutrons are about
1840 times as massive as electrons. The radius of a nucleus is of the order
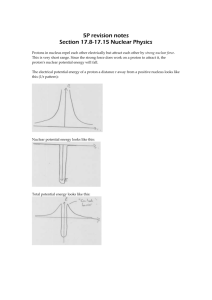
the electric potential energy of two protons is of the order or
1 MeV. Thus typical nuclear interaction energies are of the order of a few MeV, rather than a few eV as with atoms. The typical binding energy per nucleon is about
1% of the rest energy of a nucleon. For comparison, the ionization energy of the hydrogen atom is only 0.003% of the electron’s rest energy.
Angular momentum is of the same order of magnitude in both nuclei and atoms because it is determined by the value of Magnetic moments of nuclei, however, are about 1000 times smaller than those of electrons in atoms because the nuclei are so much more massive than electrons.