Ionic Bonding - Student Notes - Greer Middle College || Building the
advertisement

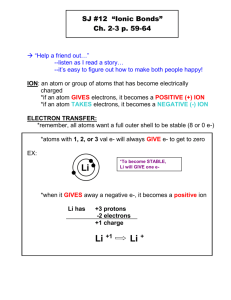
__________________ = atoms tend to gain, lose or share electrons so as to have 8 electrons C would like to Gain 4 electrons N would like to Gain 3 electrons O would like to Gain 2 electrons Electron Dot diagrams are… • A way of showing & keeping track of valence electrons. • How to write them? • Write the symbol - it represents the nucleus and inner (core) electrons • Put one dot for each valence electron (8 maximum) • They don’t pair up until they have to (Hund’s rule) X The Electron Dot diagram for Nitrogen Nitrogen has 5 valence electrons to show. First we write the symbol. Then add 1 electron at a time to each side. Now they are forced to pair up. We have now written the electron dot diagram for Nitrogen. N Electron Dot Structures Symbols of atoms with dots to represent the valence-shell electrons Learning Check A. X would be the electron dot formula for 1) Na B. X 1) B 2) K 3) Al would be the electron dot formula 2) N 3) P The type of bond can usually be calculated by finding the difference in electronegativity of the two atoms that are going together. Electronegativity Difference • If the difference in electronegativities is between: – 1.7 to 4.0: Ionic – 0.3 to 1.7: Polar Covalent – 0.0 to 0.3: Non-Polar Covalent Example: NaCl Na = 0.8, Cl = 3.0 Difference is 2.2, so this is an ionic bond! Formation of Ions from ________ Ionic compounds result when metals react with nonmetals Metals lose electrons to match the number of valence electrons of their nearest noble gas Positive ions (cations) form when the number of electrons are less than the number of protons Group 1 metals ion 1+ Group 2 metals ion 2+ • Group 13 metals ion 3+ Formation of Sodium Ion Sodium atom Na – e Sodium ion Na + e- config: e- config: 11 p+ 11 e- 11 p+ 10 e- Formation of Magnesium Ion Magnesium atom Magnesium ion Mg – e- config: 12 p+ 12 e- 2e Mg2+ e- config: 12 p+ 10 e- Electron Dots For Cations • Let’s do Scandium, #21 • The electron configuration is: 1s22s22p63s23p64s23d1 • Thus, it can lose 2e- (making it 2+), or lose 3e- (making 3+) Sc 2+ = Sc Scandium (II) ion Sc = 3+ Sc Scandium (III) ion Electron Dots For Cations • Let’s do Silver, element #47 • Predicted configuration is: 1s22s22p63s23p64s23d104p65s24d9 • Actual configuration is: 1s22s22p63s23p64s23d104p65s14d10 Ag = 1+ Ag (can’t lose any more, charges of 3+ or greater are uncommon) Learning Check A. Number of valence electrons in aluminum 1) 1 e2) 2 e3) 3 eB. Change in electrons for octet (Al) 1) lose 3e2) gain 3 e3) gain 5 eC. Ionic charge of aluminum 1) 32) 53) 3+ D. E. F. 12 p+ and 10 e1) 0 2) 2+ 50p+ and 46 e1) 2+ 2) 4+ 15 p+ and 18e2) 3+ 2) 3- 3) 23) 43) 5- Ions from __________ Ions In ionic compounds, nonmetals in 15, 16, and 17 gain electrons from metals Nonmetal add electrons to achieve the octet arrangement (forming anions) Nonmetal ionic charge: 3-, 2-, or 1- Chloride Ion unpaired electron octet :Cl e- config: 17 p+ 17 e- + e 1- : Cl: e- config: 17 p+ 18 eionic charge ________ Bond • Between atoms of metals and nonmetals with very different electronegativity • Bond formed by transfer of electrons • Produce charged ions in all states. • Conductors and have high melting point. • Examples; NaCl, CaCl2, K2O Ionic bonding • Its like taking candy from a baby……. • The nonmetal (bullies) take electrons (candy) from the metals (babies) 1). Ionic bond – electron from Na is transferred to Cl, this causes a charge imbalance in each atom. The Na becomes (Na+) and the Cl becomes (Cl-), charged particles or ions. Ionic Bonding Lets do an example by combining calcium and phosphorus: Ca P • All the electrons must be accounted for, and each atom will have a noble gas configuration (which is stable). Ionic Bonding = Ca3P2 Formula Unit This is a chemical formula, which shows the kinds and numbers of atoms in the smallest representative particle of the substance. For an ionic compound, the smallest representative particle is called a: Formula Unit Properties of Ionic Compounds 1. ____________ solids - a regular repeating arrangement of ions in the solid: Ions are strongly bonded together. – Structure is rigid. 2. High melting points • Coordination number- number of ions of opposite charge surrounding it A. Energy of Bond Formation • Lattice Energy – Energy released when one mole of an ionic crystalline compound is formed from gaseous ions – Greater lattice energy = greater ionic bond C. Johannesson Do they Conduct? • Conducting electricity means allowing charges to move. • In a solid, the ions are locked in place. • Ionic solids are insulators. • When melted, the ions can move around. 3. Melted ionic compounds conduct. – – NaCl: must get to about 800 ºC. Dissolved in water, they also conduct (free to move in aqueous solutions) - Page 198 The ions are free to move when they are molten (or in aqueous solution), and thus they are able to conduct the electric current. A. Oxidation Number • The charge on an ion. • Indicates the # of e- gained/lost to become stable. 4- 1+ 2+ 3+ 4+ 3- 2- 1(1+ to +3) 0 B. Ionic _________ Write the names of both elements, cation first. Change the anion’s ending to -ide. Write the names of polyatomic ions. For ions with variable oxidation #’s, write the ox. # in parentheses using Roman numerals. Overall charge = 0. Polyatomic Ions • “Many atoms” • A group of covalently-bonded atoms that have a net charge. • They act as a unit as an anion or cation. • You should be able to recognize polyatomic ions in a given chemical formula. • • • • • • • • • • • • • • • Symbol CH3COO1– NH41+ AsO43– C6H5COO1– HCO31– BrO31– CO32– ClO31– ClO21– C6H5O73– CN1– Cr2O72– OH1– CrO42– Poly atomic ions Name acetate ion ammonium ion arsenate ion benzoate ion bicarbonate ion bromate ion carbonate ion chlorate ion chlorite ion citrate ion cyanide ion dichromate ion hydroxide ion chromate ion Poly atomic ions • • • • • • • • • • • • • • • • • C6H5O73– CN1– Cr2O72– OH1– ClO1– IO31– PO31– NO31– NO21– C2O42– ClO41– MnO41– PO43– SiO32– SO42– SO32– S2O32– citrate ion cyanide ion dichromate ion hydroxide ion hypochlorite ion iodate ion phosphite ion nitrate ion nitrite ion oxalate ion perchlorate ion permanganate ion phosphate ion silicate ion sulfate ion sulfite ion thiosulfate ion Special Ions NAME Copper (I) Copper (II) Iron (II) Iron (III) Chromium (II) Chromium (III) Lead (II) Lead (IV) Scandium (II) Scandium (III) Ox. # +1 +2 +2 +3 +2 +3 +2 +4 +2 +3 C. Ionic ___________ Write each ion. Put the cation first. Overall charge must equal zero. • If charges cancel, just write the symbols. • If not, crisscross the charges to find subscripts. Use parentheses when more than one polyatomic ion is needed. Roman numerals indicate the oxidation #. C. Ionic Formulas potassium chloride • K+ Cl magnesium nitrate • Mg2+ NO3 copper(II) chloride • Cu2+ Cl C. Ionic Formulas calcium oxide • Ca2+ O2 aluminum chlorate • Al3+ ClO3 iron(III) oxide • Fe3+ O2 B. Ionic Names Write the names of both elements, cation first. Change the anion’s ending to -ide. Write the names of polyatomic ions. For ions with variable oxidation #’s, write the ox. # in parentheses using Roman numerals. Overall charge = 0. B. Ionic Names NaBr Na2CO3 FeCl3 Ionic hydrates • Ionic Hydrates – ionic compounds that have loosely held water molecules • Example: CuSO4●5H20(s) Formula of ionic compound raised # of water dot molecules • By heating an ionic hydrate, the water molecules are released and the ionic compound becomes “anhydrous” Example: CuSO4 ●5H20(s) + heat CuSO4 (s) (hydrated) (anhydrous) Name: ____________________________________