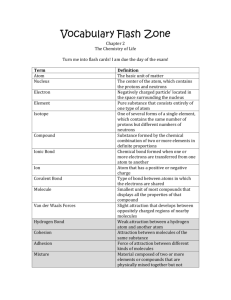
Alcohol Synthesis
advertisement

INSTRUCTION Encode the text and the structures in Chemsketch in just one file. Name the file “CHEM 32 CR Your Family Name” e.g. CHEM 32 CR Sacay.sk2. The assigned content is given on the next pages. You need to create to multiple pages for this. Please use Times New Roman size 10 for the content text. However for the structure and text part of the reactions must be Arial size 8. Before you draw the structures, you must set the structure properties given below. (eg. I saved the style as CHEM 32 style and set it as default so every time I draw the structure it will follow the same style) Some structures have colored atoms. You can change the color through the Structure Properties. You can find your assigned work, by looking for your family name on the next pages. When you find it that will be the beginning of your assigned work and ends when you find someone else’s family name. Fulgar Alkane Synthesis - Summary Alkane Synthesis - Details 1. Reduction of alkene: a. Example b. Mechanism Hydrogen gas adsorbs onto the surface of the catalyst. The hydrogen adds to the same face of the alkene producing an alkane. 2. Reduction of Alkyne: a. Example b. Mechanism Hydrogen gas adsorbs onto the surface of the catalyst. The hydrogen adds to the same face of the alkyne reducing it to an alkene. As above, the alkene can continue to react with more hydrogen to reduce the alkene to the alkane. 3. Grignard Reaction of Alkyl Halide: a. Example b. Mechanism The magnesium metal inserts itself into the C-X bond making the carbon nucleophilic and very reactive. The "carbanion" from the Grignard reagent deprotonates water to make the alkane. Alkene Synthesis - Summary Alkene Synthesis - Details 1. Dehydrohalogenation: a. Example b. Mechanism The hydroxide ion attacks an adjacent hydrogen. The C-H bond is broken, and the pair of electrons becomes part of the double bond while ejecting the bromide ion. 2. Dehydration: a. Example b. Mechanism The -OH group of the alcohol is protonated by the acid. A water molecule leaves resulting in the formation of a carbocation intermediate. A water molecule takes an adjacent proton, and the C-H bond breaks. The pair of electrons on C become the double bond which quenches the positive charge on the adjacent C atom. Alkyne Synthesis - Summary Alkyne Synthesis - Details 1. Didehydrohalogenation of vinylic dihalide: a. Example b. Mechanism The hydroxide ion removes a proton, the electrons in that bond form the bouble bond, and adjacent halogen is ejected. The process is repeated with the resulting vinylic halide. 2. Dehydrohalogenation of vinylic halide: a. Example b. Mechanism The hydroxide ion removes a proton, the electrons in that bond form the bouble bond, and adjacent halogen is ejected. 3. Halogenation / didehydrohalogenation of alkene: a. Example b. Mechanism The alkene is halogenated (see Alkene Reaction Details) first. The second step involves didehydrohalogenation (see Alkyne Synthesis Details). 4. Dehydrohalogenation / halogenation / didehydrohalogenation of alkyl halide: a. Example b. Mechanism The alkyl halide is dehydrohalogenated (see Alkene Synthesis Details) first. The alkene is halogenated (see Alkene Reaction Details) first. The second step involves didehydrohalogenation (see Alkyne Synthesis Details). 5. DAlkylation of terminal alkyne: a. Example b. Mechanism The hydrogen attached to the alkyne carbon as fairly acidic (in general terms of alkane acidity), and can be removed by treatment with strong base (sodium amide in this case). Treatment of the resulting acetylide anion with an alkyl halide results in the formation of a carbon-carbon bond. Note: This sequence converts a terminal alkyne into an internal alkyne. The reaction is very useful in terms of making longer carbon chains. The alkyl halide in the reaction should be 1o or 2o in order for the reaction to work as advertised. Alkane Reactions - Summary Reactions of Alkanes 1. Addition of halide (X2): a. Example b. Mechanism Halogenation of an alkane proceeds through a radical reaction pathway composed of essentially three steps: initiation, propagation, and termination. In initiation, radicals are produced by the homolytic bond cleavage of the halogen molecule. Propagation involves abstraction of a hydrogen atom from the alkane producing an alkyl radical. The alkyl radical can then react with more halogen molecule to produce the product and a new halogen radical. This process is self-sustaining and referred to as a chain reaction. Product is continuously produced until termination steps occur. In termination, radicals react with each other to produce products where no new radicals are formed. This will eventually stop the reaction. The major drawback to this type of reaction is that a mixture of halogenated products is usually produced. For this reason, this reaction is not synthetically useful. Sacay Alkene Reactions - Summary Addition Reactions: Oxidative Cleavage Reactions: Related Reaction - Oxidative Cleavage of 1,2-diols: Reactions of Alkenes 1. Addition of hydrogen halide (HX): a. Example b. Mechanism Addition of HX follows Markovnikov's Rule: the carbon with the most hydrogen atoms attached gets the hydrogen. This rule is followed because the most stable carbocation intermediate will be formed. The carbocation is now sp2 hybridized with an empty p-orbital which can accept electrons from a nucleophile (Cl- in this case). Note: Attack of the nucleophile can come from above or below with the concomitant stereochemical consequences. 2. Addition of halogens (X2): a. Example b. Mechanism Addition of halogen to an alkene is possible because of the polarizability of the halogen. As the halogen approaches the site of high electron density (the double bond), the bromine molecule is polarized such that the nearest bromine atom now has some partial positive charge. The electrons attack this bromine atom breaking the Br-Br bond. The carbocation intermediate formed is stabilized by non-bonding electrons donated by the adjacent bromine atom. This non-classical carbocation is referred to as a bromonium ion intermediate (the generic term is halonium ion, and the halogen name is used for specific a particular halogen). The bromonium ion shields the one face of the molecule from attack by a nucleophile; attack must come from the side opposite of the bromonium ion. The net result is anti-addition of halogen to the double bond. Note: The nucleophile will attack the carbon atom of the bromonium ion intermediate most able to bear positive charge. In this case, that is the carbon atom with the methyl substituent (it is a 3o carbon atom). 3. Halohydrin formation: a. Example b. Mechanism Halohydrin formation is analogous to the addition of halogen to an alkene with two changes: the nucleophile in this case is a water molecule, and a final deprotonation is required to make the neutral alcohol. Note: Since a bromonium ion intermediate is formed, we again find antistereochemistry in the product. Also, the nucleophile (water) will again attack the more highly-substituted carbon atom of the bromonium ion intermediate. 4. Hydration: a. Example b. Mechanism Hydration of an alkene with aqueous acid follows Markovnikov's Rule. The mechanism is very similar to the addition of hydrogen halide (see above) with two exceptions: the nucleophile is water, and an additional deprotonation step is required to make the neutral alcohol. Note: This reaction requires the use of strong acid and high heat. It is an industrially important reaction, but not of much synthetic use in the lab as many organic compounds are adversely affected by these conditions. 5. Hydration via oxymercuration: a. Example b. Mechanism Oxymercuration/demercuration involves mercuric acetate as the electrophile in the reaction. An intermediate mercurinium ion reminiscent of the halonium ion forms, and then goes on to react with the nucleophile water. Note: The nucleophile (water) will attack the more highly-substituted carbon atom of the mercurinium ion intermediate. Deprotonation yields the neutral alcohol - organomercury product. Reduction with sodium borohydride yields the alcohol. Sodium borohydride effectively replaces the mercury atom where it is in the molecule with hydrogen. You are not responsible for this part of the mechanism. This can be thought of as the Markovnikov product, because the carbon with the most hydrogens will ultimately get the hydrogen from the sodium borohydride reduction. 6. Hydration via hydroboration: a. Example b. Mechanism Hydroboration of an alkene involves the reaction of an alkene with borane. The boron atom has an empty p-orbital which acts as an electrophile toward the double bond. Hydride transfer from borane to the other carbon atom of the double bond occurs simultaneously with bond formation to boron. A four-atom transition state closely describes the bond-breaking and bond-forming interactions. Because borane has three hydrogen atoms attached, each borane molecule reacts with three alkenes to form a trialkyl borane. Hydrogen peroxide oxidizes the boron atom, and the carbon-boron bonds are replaced with carbon-oxygen bonds. You are not responsible for this portion of the mechanism. Note: The product exhibits anti-Markovnikov addition since the more highlysubstituted carbon atom gets the hydrogen. Remember, that the boron and hydrogen add from the same side of the alkene, and the boron is replaced where it is in the molecule with oxygen. Therefore, the -H and -OH are added syn to each other. 7. Hydrogenation: a. Example b. Mechanism Hydrogen gas adsorbs onto the surface of the catalyst. The hydrogen adds to the same face of the alkene producing an alkane. 8. Dihydroxylation: a. Example b. Mechanism Dihydroxylation occurs via the reaction of an alkene with osmium tetroxide. A cyclic osmate intermediate is formed which is cleaved in a second separate step with sodium bisulfite. Note: You are not responsible for this mechanism. However, be aware that the two hydroxyl groups are added to the same side of the alkene resulting in syn stereochemistry. 9. Oxidative cleavage of 1,2-diols: a. Example b. Mechanism You are not responsible for this mechanism. However, realize that a 1,2-diols are oxidatively cleaved to form a dicarbonyl compound. Note: If the 1,2-diol is part of an open chain molecule, then two carbonyl compounds are produced. If the diol is part of a ring system (as in this example, then a single dicarbonyl compound is produced. 10. Ozonolysis: a. Example b. Mechanism Ozonolysis is very complicated mechanistically, but the key points involve the formation of a molozonide intermediate which rearranges to an ozonide. Ozonides are very explosive, and are never isolated. Instead, they are treated with a reducing agent (Zn) to convert them to carbonyl compounds. You are not responsible for this mechanism, however, be aware of the result: an alkene is converted into a dicarbonyl compound. Note: If the alkene is part of an open chain molecule, then two carbonyl compounds are produced. If the alkene is part of a ring system (as in this example, then a single dicarbonyl compound is produced. The net result of this reaction is the same as for the oxidative cleavage of 1,2-diols. 11. Oxidation by potassium permanganate: a. Example b. Mechanism No mechanism is required for this reaction. Potassium permanganate oxidative cleavage of an alkene is a very complicated chemical reaction. However, you must be aware of the result: the carbon-carbon double bond is replaced with a carbon-oxygen double bond. Additionally, all vinylic hydrogen atoms are replaced with -OH groups Note: In the case of a terminal alkene, the terminal carbon atom is removed as carbon dioxide. This is a chain-shortening reaction (see top reaction above). This reaction works best for symmetrical alkenes, because, in this case, only one product is produced (see middle example above). For unsymmetrical alkenes mixtures are formed (see bottom example above). 12. Simmons-Smith reaction: The Simmons-Smith reaction is related to the dichlorocarbene reaction. We will show an example of each and study the mechanism of the dichlorocarbene reaction. The mechanism for the Simmons-Smith reaction is not as straight forward, but the end result is the same as for the dichlorocarbene reaction. a. Example b. Mechanism The dichlorocarbene reaction involves the decomposition of chloroform with base. The hydrogen in chloroform is fairly acidic and is abstracted by hydroxide ion. The carbanion initially formed decomposes to form a carbene (a neutral carbon species with an unshared pair of electrons in an sp3 hybrid orbital and an unoccupied p-orbital). The carbene is both nucleophilic (lone pair) and electrophilic (empty p-orbital) and can react with alkenes to form dichlorocyclopropane rings. Note: The Simmons-Smith reaction is very similar, but produces cyclopropane rings, which are more synthetically useful than the dichloro counterpart produced in the dichlorocarbene reaction. Alkyne Reactions - Summary Antonio Reactions of Alkynes 1. Addition of hydrogen halide (HX): a. Example b. Mechanism This reaction is very similar to alkene hydrohalogenation. With one equivalent of hydrogen halide, this reaction can be stopped at the production of the vinylic halide. If an excess of hydrogen halide is used, then the geminal dihalide is produced. 2. Addition of halide (X2): a. Example b. Mechanism test 3. Hydration: a. Example b. Mechanism Mercury (II) ion is the elctrophile in this reaction. It interacts with the less highly substituted carbon atom of the triple bond. The resulting vinylic carbocation is attacked by water, which is subsequently deprotonated. The mercury ion is replaced by acidic hydrogen to form an enol intermediate. The enol tautomerizes to the more stable ketone. Note: Hydration of terminal alkynes always leads to the production of methyl ketones. Symmetrical alkynes lead to only one ketone product, whereas unsymmetrical internal alkynes lead to mixtures of ketones. 4. Hydroboration: a. Example b. Mechanism Hydroboration of an alkyne involves the reaction of an alkyne with borane. The boron atom has an empty p-orbital which acts as an electrophile toward the triple bond. Hydride transfer from borane to the other carbon atom of the triple bond occurs simultaneously with bond formation to boron. A four-atom transition state closely describes the bond-breaking and bond-forming interactions. Because borane has three hydrogen atoms attached, each borane molecule reacts with three alkynes to form a trialkenyl borane. Hydrogen peroxide oxidizes the boron atom, and the carbon-boron bonds are replaced with carbon-oxygen bonds. You are not responsible for this portion of the mechanism. The resulting enol tautomerizes to the more stable ketone. Note: Hydration of terminal alkynes always leads to the production of methyl ketones. Symmetrical alkynes lead to only one ketone product, whereas unsymmetrical internal alkynes lead to mixtures of ketones. 5. Oxidative cleavage with potassium permanganate: a. Example b. Mechanism No mechanism is required for this reaction. Potassium permanganate oxidative cleavage of an alkyne is a very complicated chemical reaction. However, you must be aware of the result: each carbon of the carbon-carbon triple bond is oxidized up to the carboxylic acid. Additionally, all acetylenic hydrogen atoms are replaced with -OH groups. Note: In the case of a terminal alkyne, the terminal carbon atom is removed as carbon dioxide. This is a chain-shortening reaction (see top reaction above). This reaction works best for symmetrical alkynes, because, in this case, only one product is produced (see middle example above). For unsymmetrical alkynes mixtures are formed (see bottom example above). 6. Hydrogenation to alkane: a. Example b. Mechanism Hydrogen gas adsorbs onto the surface of the catalyst. The hydrogen adds to the same face of the alkyne reducing it to an alkene. The alkene can continue to react with more hydrogen to reduce the alkene to the alkane. 7. Hydrogenation to (Z)-alkene: a. Example b. Mechanism Hydrogen gas adsorbs onto the surface of the catalyst. The hydrogen adds to the same face of the alkyne reducing it to an alkene. The catalyst has been deactivated by treatment with lead acetate and quinone, and is thus unreactive toward alkenes. The reaction stops at the production of the (Z)-alkene 8. Hydrogenation to (E)-alkene: a. Example b. Mechanism This reaction is often referred to as a "solvated electron" reaction. Lithium metal donates an electron to the alkyne forming a radical anion that is protonated by the solvent. Lithium metal again donates an electron to the vinylic radical to form a new anion which is again protonated by solvent. Note: The trans-stereochemistry is determined when the more stable transvinylic anion is formed. Equilibrium is not established before protonation occurs, therefore, the trans- product is isolated. Alkenes are much less reactive under these conditions, so the alkene product is isolable. Aromatic Reactions - Summary Reactions of Aromatics 1. Addition of halogen (X2): a. Example b. Mechanism Step 1: Step 2: Step 1: The Lewis acid catalyst iron(III) bromide polarizes the Br-Br bond to produce a nucleophilic bromine species. Step 2: A pair of pi electrons from the aromatic ring attacks the electron-poor bromine atom to form a non-aromatic intermediate. A base in solution deprotonates this intermediate to restore aromaticity and form the product. Note: The aromatic halogenation reaction provides a route to the halogenated aromatic compounds and also serves as a precursor to nucleophilic aromatic substitution reactions via the benzyne intermediate. 2. Nitration: a. Example b. Mechanism Step 1: Step 2: Step 1: Sulfuric acid protonates nitric acid. The protonated form of nitric acid decomposes to form the nitronium ion which contains an electrophilic nitrogen atom. Step 2: A pair of pi electrons from the aromatic ring attacks the nitrogen atom to form a non-aromatic intermediate. A base in solution deprotonates this intermediate to restore aromaticity and form the product. Note: The aromatic nitration reaction is particularly useful because the nitrosubstituted product can be reduced to the amino group by reagents such as SnCl2 or Fe. This two-step nitration and reduction is a key part of the synthesis of dyes and other pharmaceutical agents. 3. Sulfonation: a. Example b. Mechanism Step 1: Step 2: Step 1: Sulfuric acid protonates sulfur trioxide. The protonated form of sulfur trioxide contains an electrophilic sulfur atom. Step 2: A pair of pi electrons from the aromatic ring attacks the sulfur atom to form a non-aromatic intermediate. A base in solution deprotonates this intermediate to restore aromaticity and form the product. Note: The aromatic sulfonation reaction is particularly useful because the sulfonic acid products are valuable intermediates in the preparation of dyes and other pharmaceutical agents. Sulfa drugs, the first effective antibiotics, were made via this route. 4. Friedel-Crafts Acylation: a. Example b. Mechanism Step 1: Step 2: Step 1: Aluminum chloride, a Lewis acid catalyst, reacts with the acid chloride to form an acyl cation. This is the source of the electrophilic carbon for the reaction. Step 2: A pair of pi electrons from the aromatic ring attacks the carbon atom to form a non-aromatic intermediate. A base in solution deprotonates this intermediate to restore aromaticity and form the product. Note: The Friedel-Crafts acylation is a very synthetically useful reaction because it provides a mechanism for making carbon-carbon bonds. The products of these reactions also contain the carbonyl functionality which is also synthetically very useful. There are several limitations to the Friedel-Crafts acylation reaction outlined below: 1. Aromatic rings with the amino functionality cannot be acylated in this manner. 2. Highly deactivated aromatic rings (e.g. nitrobenzene) cannot be acylated. 5. Hydrogenation: a. Example b. Mechanism Step 1: Step 2: Step 1: Hydrogen gas adsorbs to the surface of the Rh/C catalyst surface. Step 2: A pair of pi electrons from the aromatic ring attacks an adsorbed hydrogen atom while simultaneously bonding to a hydride atom from the catalyst surface. The process continues until all pi electrons are reduced. Note: Hydrogenation of aromatic compounds leads to cyclohexane derivatives. 6. Friedel-Crafts Alkylation: a. Example b. Mechanism Step 1: Step 2: Step 1: Aluminum chloride, a Lewis acid catalyst, reacts with the alkyl chloride to form a carbocation. This is the source of the electrophilic carbon for the reaction. Step 2: A pair of pi electrons from the aromatic ring attacks the carbocation to form a non-aromatic intermediate. A base in solution deprotonates this intermediate to restore aromaticity and form the product. Note: The Friedel-Crafts alkylation is a synthetically useful reaction because it provides a mechanism for making carbon-carbon bonds. There are several limitations to the Friedel-Crafts alkylation reaction outlined below: 1. Aromatic rings with the amino functionality cannot be alkylated in this manner. 2. Highly deactivated aromatic rings (e.g. nitrobenzene) cannot be alkylated. 3. Carbocation rearrangement can occur. 4. Over-alkylation can occur under normal reaction conditions (alkyl groups activate the ring to further EAS). Barcelon SN2 - Substitution Nucleophilic 2nd Order Why do we call this an SN2 reaction? substitution: one group substituted for another (CN- for Br- in this case) nucleophilic: attacking group is a nucleophile (CN-) 2nd order: rate of reaction depends on the concentration of both the nucleophile and the substrate. The slowest step in a reaction sequence determines the overall rate of reaction. Since this is a one step mechanism, this is the slow step, and therefore controls the rate of reaction. Rate = k [nucleophile] [substrate] * Note that the SN2 reaction involves inversion of the carbon atom undergoing reaction. Reaction Diagram: Factors Influencing the SN2 Reaction The Substrate: The bulkier the groups attached to the carbon atom undergoing reaction, the slower the reaction will be. In fact, the order of reactivity for substituted carbon atoms is: The Nucleophile: The more nucleophilic the attacking species is, the faster the reaction will proceed. Nucleophilicity increases with: basicity - the more basic the species is, the more nucleophilic it is. period - as you move down the periodic table nucleophilicty increases: I- > Br- > Cl- > F- charge - negatively charged species are more nucleophilic than their neutral counterparts: OH- > H2O The Leaving Group The best leaving groups are those most able to bear negative charge (remember, the leaving group will be taking the electrons with which it was once bonded to carbon). Therefore, the weaker the base, the better the leaving group. The leaving group order is as follows: The Solvent Polar protic solvents (such as methanol or ethanol) tend to solvate the nucleophile, thereby lowering its ground-state energy, increasing G (of the transition state), and decreasing the reaction rate. Polar aprotic solvents (such as DMSO, DMF, and HMPA) tend to solvate the metal cation counterpart of the nucleophile, effectively creating a "naked" nucleophile. This raises the nucleophiles ground-state energy, decreasing G (of the transition state), and increasing the reaction rate. The structures for the common polar aprotic solvents follow: SN1 - Substitution Nucleophilic 1st Order Why do we call this an SN1 reaction? substitution: one group substituted for another (HO for Br- in this case) nucleophilic: attacking group is a nucleophile (H2O) 1st order: rate of reaction depends only on the concentration of the substrate. The slowest step in a reaction sequence determines the overall rate of reaction. Since the ionization step is the slow step, this controls the rate of reaction. Rate = k [substrate] * Note that the SN1 reaction involves racemization of the carbon atom undergoing reaction. Reaction Diagram: Factors Influencing the SN1 Reaction The Substrate: Those substrates that yield the most stable carbocations will react fastest. In fact, the order of reactivity for substituted carbon atoms is: The Nucleophile: The nucleophile must be non-basic to prevent competitive elimination reactions (see E1 Reactions page), but otherwise does not affect the reaction. Neutral nucleophiles work well. The Leaving Group The best leaving groups are those most able to bear negative charge (remember, the leaving group will be taking the electrons with which it was once bonded to carbon). Therefore, the weaker the base, the better the leaving group. The leaving group order is as follows: Since SN1 reactions are often carried out in acidic conditions, water can be a good leaving group (usually formed from protonated alcohols). The Solvent Polar protic solvents (such as methanol, ethanol, or water) tend to solvate the nucleophile, but will solvate the carbocation intermediate even better. Solvation of the nucleophile lowers its ground-state energy, however, solvation of the carbocation intermediate lowers its energy even more. The net effect is to decrease G (of the transition state), which increases the reaction rate. E2 - Elimination 2nd Order Why do we call this an E2 reaction? elimination: two groups are removed from adjacent carbon atoms (H+ from one carbon and Cl- from the other carbon) to form a double bond. 2nd order: rate of reaction depends on the concentration of both the base and the substrate. The slowest step in a reaction sequence determines the overall rate of reaction. Since this is a one step mechanism, this is the slow step, and therefore controls the rate of reaction. Rate = k [base] [substrate] * Note that the E2 reaction produces the most stable alkene possible. This is called Zaitsev's rule. In general, the more highly substituted the double bond, the more stable. Also, remember that trans- double bonds are more stable than cis- double bonds. * Newman projections are very useful for determining the geometry around the double bond that is forming, and is also very useful in helping to determine which of the available adjacent hydrogen atoms is most likely to be abstracted by the base. Predicting the Outcome of E2 Reactions In the following example which adjacent hydrogen is most likely to be abstracted (Ha or Hb), and what is the most likely geometry going to be for the double bond which is formed? The best way to answer this question is to draw the Newman projection for the substrate and rotate the projection to make all of the adjacent hydrogens anti periplanar to the leaving group (Br in this case). Then choose the Newman projection that places the bulkiest groups as far apart as possible. This is the conformation most likely to undergo reaction. The Newman projection on the right has the anti periplanar conformation necessary for the E2 reaction and also places the bulky phenyl groups as far apart as possible. This is the conformation that will lead to reaction and produce a trans- double bond. Sometimes, there are hydrogen atoms on two different adjacent carbon atoms that can be abstracted to form alkenes in an E2 reaction. In general, pick the hydrogen atoms on the more highly substituted carbon atom, as this will lead to the more highly substituted alkene product. In the following example which adjacent hydrogen is most likely to be abstracted (Ha, Hb, or Hc), and what is the most likely geometry going to be for the double bond which is formed? Hopefully, it should be obvious that the Hc hydrogens, if abstracted, would lead to a mono-substituted alkene. Therefore, only Ha or Hb are likely to be removed. Again, use Newman projections to answer the question of which one is more likely to be removed. E2 Reactions and Cyclohexane Conformations The anti periplanar geometry is particularly important in cyclohexane rings, where the chair conformation forces a rigid relationship between the substituents on neighboring carbon atoms. Remember, that there are two possible chair conformations that a cyclohexane ring can adopt. You must consider only that chair conformation which has the leaving group in the axial position, as it is that conformation that allows an anti periplanar orientation between the leaving group and a neighboring hydrogen atom. As before, to determine which hydrogen atom is most likely to be abstracted, Newman projections prove invaluable. For the following example, which hydrogen, Ha or Hb is likely to be removed, and what is the most likely alkene product to be formed? The conformation shown will not react via an E2 mechanism, so the ring-flipped conformation is drawn instead. It should then be obvious that only Hb is anti periplanar to the leaving group, and is the only hydrogen that can be removed in the reaction. Factors Influencing the E2 Reaction The Substrate: E2 reactions can occur with 1o, 2o, and 3o alkyl halides, but works best with 2o and 3o alkyl halides. The E2 reaction will be favored when strong bases are used in the reaction. The Leaving Group The best leaving groups are those most able to bear negative charge (remember, the leaving group will be taking the electrons with which it was once bonded to carbon). Therefore, the weaker the base, the better the leaving group. The leaving group order is as follows: E1 - Elimination 1st Order Why do we call this an E1 reaction? elimination: two groups are removed from adjacent carbon atoms (H+ from one carbon and Cl- from the other carbon) to form a double bond. 1st order: rate of reaction depends only on the concentration of the substrate. The slowest step in a reaction sequence determines the overall rate of reaction. Since the ionization step is the slow step, this controls the rate of reaction. Rate = k [substrate] * Note that the E1 reaction produces the most stable alkene possible. This is called Zaitsev's rule. In general, the more highly substituted the double bond, the more stable. Also, remember that trans- double bonds are more stable than cis- double bonds. Competition Between E1 and SN1 Reactions E1 reactions begin with the same unimolecular ionization step seen in the SN1 mechanism, but the ionization is followed by loss of H+ from the intermediate carbocation rather than by substitution. In fact, in a protic solvent with a nonbasic nucleophile, both E1 and SN1 occur in competition with each other and mixtures of products are formed. Factors Influencing the E2 Reaction The Substrate: E1 reactions can occur only with 2o (allylic or benzylic) and 3o alkyl halides, since these substrates will form the most stable carbocations after ionization. The Leaving Group The best leaving groups are those most able to bear negative charge (remember, the leaving group will be taking the electrons with which it was once bonded to carbon). Therefore, the weaker the base, the better the leaving group. The leaving group order is as follows: Bragais Alcohol Synthesis From Alkenes From Carbonyl Compounds From Grignard Reaction Alcohol Formation: Halohydrin Example Mechanism Halohydrin formation is analogous to the addition of halogen to an alkene with two changes: the nucleophile in this case is a water molecule, and a final deprotonation is required to make the neutral alcohol. Note: Since a bromonium ion intermediate is formed, we again find anti-stereochemistry in the product. Also, the nucleophile (water) will again attack the more highly-substituted carbon atom of the bromonium ion intermediate. Alcohol Formation: Oxymercuration Example Mechanism Oxymercuration/demercuration involves mercuric acetate as the electrophile in the reaction. An intermediate mercurinium ion reminiscent of the halonium ion forms, and then goes on to react with the nucleophile water. Note: The nucleophile (water) will attack the more highly-substituted carbon atom of the mercurinium ion intermediate. Deprotonation yields the neutral alcohol / organomercury product. Reduction with sodium borohydride yields the alcohol. Sodium borohydride effectively replaces the mercury atom where it is in the molecule with hydrogen. You are not responsible for this part of the mechanism. This can be thought of as the Markovnikov product, because the carbon with the most hydrogens will ultimately get the hydrogen from the sodium borohydride reduction. Alcohol Formation: Hydroboration Example Mechanism Hydroboration of an alkene involves the reaction of an alkene with borane. The boron atom has an empty p-orbital which acts as an electrophile toward the double bond. Hydride transfer from borane to the other carbon atom of the double bond occurs simultaneously with bond formation to boron. A fouratom transition state closely describes the bond-breaking and bond-forming interactions. Because borane has three hydrogen atoms attached, each borane molecule reacts with three alkenes to form a trialkyl borane. Hydrogen peroxide oxidizes the boron atom, and the carbon-boron bonds are replaced with carbon-oxygen bonds. You are not responsible for this portion of the mechanism. Note: The product exhibits anti-Markovnikov addition since the more highly-substituted carbon atom gets the hydrogen. Remember, that the boron and hydrogen add from the same side of the alkene, and the boron is replaced where it is in the molecule with oxygen. Therefore, the -H and OH are added syn to each other. Alcohol Formation: Dihydroxylation Example Mechanism Dihydroxylation occurs via the reaction of an alkene with osmium tetroxide. A cyclic osmate intermediate is formed which is cleaved in a second separate step with sodium bisulfite. Note: You are not responsible for this mechanism. However, be aware that the two hydroxyl groups are added to the same side of the alkene resulting in syn stereochemistry. Alcohol Formation: Reduction of Aldehyde Example Mechanism Reduction of an aldehyde with sodium borohydride will always yield a primary alcohol. Sodium borohydride is a source of hydride ion (H-). The reagent delivers hydride ion to the carbonyl carbon at the same time that the boron is forming a Lewis acid interaction with the carbonyl oxygen. The oxygen boron bond is cleaved with acid to form the alcohol. Note: Although lithium aluminum hydride will also work for aldehydes, it is seldom employed because of the dangers involved in working with this reagent. Alcohol Formation: Reduction of Ketone Example Mechanism Reduction of a ketone with sodium borohydride will always yield a secondary alcohol. Sodium borohydride is a source of hydride ion (H-). The reagent delivers hydride ion to the carbonyl carbon at the same time that the boron is forming a Lewis acid interaction with the carbonyl oxygen. The oxygen boron bond is cleaved with acid to form the alcohol. Note: Although lithium aluminum hydride will also work for ketones, it is seldom employed because of the dangers involved in working with this reagent. Alcohol Formation: Reduction of Ester Example Mechanism Reduction of an ester with lithium aluminum hydride will always yield a primary alcohol. Lithium aluminum hydride is a source of hydride ion (H-). The reagent delivers hydride ion to the carbonyl carbon at the same time that the aluminum is forming a Lewis acid interaction with the carbonyl oxygen. The oxygen aluminum bond is cleaved when the ester group is transferred to the aluminum while reforming the carbon oxygen double bond. As an intermediate, an aldehyde is formed. The aldehyde can not be isolated, however, because the remaining lithium aluminum hydride can readily reduce the aldehyde. The net result is the transfer of two equivalents of hydride ion to the carbonyl carbon. Note: Sodium borohydride is sufficiently mild that it is not effective in reducing the ester. Alcohol Formation: Reduction of Acid Example Mechanism Reduction of an acid with lithium aluminum hydride will always yield a primary alcohol. Lithium aluminum hydride is a source of hydride ion (H-). The reagent delivers hydride ion to the carbonyl carbon at the same time that the aluminum is forming a Lewis acid interaction with the carbonyl oxygen. The oxygen aluminum bond is cleaved when the hydroxyl group is transferred to the aluminum while reforming the carbon oxygen double bond. As an intermediate, an aldehyde is formed. The aldehyde can not be isolated, however, because the remaining lithium aluminum hydride can readily reduce the aldehyde. The net result is the transfer of two equivalents of hydride ion to the carbonyl carbon. Note: Sodium borohydride is sufficiently mild that it is not effective in reducing the acid. Alcohol Formation: Grignard with Aldehyde Example Mechanism Reaction of a Grignard reagent with an aldehyde will always form a secondary alcohol. The magnesium of the Grignard forms a Lewis acid-base complex with the carbonyl oxygen of the aldehyde, thereby making the carbonyl carbon a better electron-pair acceptor. Nucleophilic addition of the alkyl group of the Grignard reagent produces a tetrahedral magnesium alkoxide intermediate. This undergoes hydrolysis in aqueous acid. Note: The Grignard reagent is extremely basic. For this reason, the reaction will not work with molecules containing the following functional groups: alcohols, amines, thiols, and carboxylic acids. The Grignard reagent is protonated by these functional groups. Additionally, Grignard reagents cannot be prepared from alkyl halides containing the following functional groups: aldehydes, ketones, amides, nitriles, esters, and sulfones. The Grignard reagent will add to these groups. Alcohol Formation: Grignard with Ketone Example Mechanism Reaction of a Grignard reagent with an ketone will always form a tertiary alcohol. The magnesium of the Grignard forms a Lewis acid-base complex with the carbonyl oxygen of the ketone, thereby making the carbonyl carbon a better electron-pair acceptor. Nucleophilic addition of the alkyl group of the Grignard reagent produces a tetrahedral magnesium alkoxide intermediate. This undergoes hydrolysis in aqueous acid. Note: The Grignard reagent is extremely basic. For this reason, the reaction will not work with molecules containing the following functional groups: alcohols, amines, thiols, and carboxylic acids. The Grignard reagent is protonated by these functional groups. Additionally, Grignard reagents cannot be prepared from alkyl halides containing the following functional groups: aldehydes, ketones, amides, nitriles, esters, and sulfones. The Grignard reagent will add to these groups. Alcohol Formation: Grignard with Ester Example Mechanism Reaction of a Grignard reagent with an ester will always form a tertiary alcohol. The magnesium of the Grignard forms a Lewis acid-base complex with the carbonyl oxygen of the ester, thereby making the carbonyl carbon a better electron-pair acceptor. Nucleophilic addition of the alkyl group of the Grignard reagent produces a tetrahedral magnesium alkoxide intermediate. This intermediate decomposes into a ketone when the carbon oxygen double bond is reformed and the -OR group of the ester leaves as an alkoxide ion. Since a ketone is formed as an intermediate, it continues to react with more Grignard reagent to form a second tetrahedral magnesium alkoxide intermediate. This undergoes hydrolysis in aqueous acid. The net result is the addition of two equivalents of the Grignard reagent to the ester. Note: The Grignard reagent is extremely basic. For this reason, the reaction will not work with molecules containing the following functional groups: alcohols, amines, thiols, and carboxylic acids. The Grignard reagent is protonated by these functional groups. Additionally, Grignard reagents cannot be prepared from alkyl halides containing the following functional groups: aldehydes, ketones, amides, nitriles, esters, and sulfones. The Grignard reagent will add to these groups. Nolasco Aldehyde Synthesis Summary Ketone Synthesis Summary Carboxylic Acid Synthesis Summary Carboxylic Acid Derivative Synthesis Summary Amine Synthesis Summary Aldehyde/Ketone Reactions Aldehyde Only Aldehyde & Ketone -Substitution Reactions Enols (neutral) Related Reaction Enolate Ions Direct Alkylation Malonic Ester Synthesis Acetoacetic Ester Synthesis Carbonyl Condensation Aldol Condensation Reactions Claisen Condensation Reactions Conjugate Addition Condensation Reactions Carboxylic Acid Reactions Summary Carboxylic Acid Derivative Reactions Acid Halides Acid Anhydrides Esters Amides Nitriles Amine Reactions Alkyl Amines Aryl Amines