Atomic_spectra
advertisement

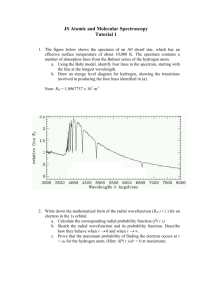
Atomic Spectra & The Periodic Properties Of Elements Objectives 1. Determine the emission spectrum of Hydrogen and other elements. 2. Calculate the expected wavelengths of H using the Rydberg equation. 3. Discover the relationship between the chemical reactivity of various elements and their positions on the periodic table. Animation of the dispersion of white light as it travels through a triangular prism. History of Optics & Light Studies Ibn Alhazen is considered the “Father of Optics” He wrote the “Book of Optics”, which correctly explained and proved the modern theory of vision. His experiments on optics greatly influenced later scientists. His experiments included ones on lenses, mirrors, refraction, reflection, and the dispersion of light into its constituent colors. He studied the electromagnetic aspects of light, and argued that rays of light are streams of energy particles traveling in straight lines. Ibn Alhazen (965 – 1039) Arab Muslim Scientist “Father of Optics” Historical Background of Spectroscopy In 1608, Galileo Galilei is credited as the first to turn his telescope to the heavens. He soon discovered craters on our Moon, sun spots, the moons of Jupiter, and that Venus has phases like our Moon. Galileo claimed that his observations only made sense if all the planets revolved around the Sun (as proposed by Aristarchus and Copernicus) rather than the Earth. The Inquisition eventually forced Galileo to publicly recant this conclusion. Galileo Galilei 1564 - 1642 A Quantitative Study of Light Sir Isaac Newton was one of the first people to study light scientifically. In 1672, Newton directed a beam of white light through a triangular bar of glass, called a “prism”. He discovered that the light coming out of the prism was separated into bands of colors. The arrangement of colors produced by a prism is called a “spectrum”. Sir Isaac Newton 1643 - 1727 Prior to this it was believed that “white light” was equal to purity. Original Studies Of Light Used Only One Prism . When a narrow band of light from a “white” light source is sent through a prism, a continuous spectrum containing all wavelengths of visible light is formed. Newton’s Contribution to Spectroscopy Newton contributed more to spectroscopy than scientifically proving that sunlight traveling through a prism was always broken down into the components of the rainbow. In fact, his main contribution was to show that after the sunlight had been broken down into its components by one prism, if a narrow ray of the light from the first prism was passed through another prism no further breakdown of light occurred. Classification of Electromagnetic Radiation The color components of light are separated along the visible range of light. The visible range of light (400-700 nm) is merely a small portion of the entire electromagnetic spectrum. Advancements in the Study of Light Joseph von Fraunhofer discovered the dark absorption lines in the Sun's spectrum (known as Fraunhofer lines) and designed achromatic telescope objectives. At age 11, he was orphaned and forced to apprentice for no pay with a harsh glassmaker named Philipp Anton Weichelsberger. In 1801, the glass shop collapsed and Fraunhofer was buried alive. Joseph von Fraunhofer (March 6, 1787 – June 7, 1826) German Optician When Fraunhofer survived the collapse, the court-councilor von Utzschneider, gave him books on mathematics and optics. King Max Joseph gave him a present of eighteen ducats. With this money Joseph acquired his own glass grinding machine and bought his release from Weichelsberger. Development of the Spectroscope Joseph von Fraunhofer’s initial desire was to create a glass lens that did not produce an image that was fringed with a rainbow of colors. He realized the problem was that the glass lens bent some colors more than others. He began searching for a source of light of a single color. Joseph von Fraunhofer (March 6, 1787 – June 7, 1826) In 1814, he developed a spectroscope to study the spectrum of the light given off by the sun. He was amazed to discover that in the midst of the rainbow of colors was a series of black lines. These dark lines were later determined to be the result of the absorption of selected frequencies of the electromagnetic radiation by an atom or a molecule. Fraunhofer lines observable in the Solar Spectrum 390 nm 700 nm Development of Diffraction Gratings Fraunhofer also completed an important theoretical work on diffraction and established the laws of diffraction. One important innovation that Fraunhofer made was to place a diffraction slit in front of the objective of a measuring telescope in order to study the solar spectrum. He later made and used diffraction gratings with up to 10,000 parallel lines per inch. By means of these gratings he was able to measure the minute wavelengths of the different colors of light. (Diffraction gratings will be discussed more later.) 1855-1860 - Gustav Kirchhoff and Robert Bunsen Gustav Robert Kirchhoff Robert Wilhelm Eberhard Bunsen (March 12, 1824 – October 17, 1887) German Physicist (March 31, 1811 – August 16,1899) German Chemist Bunsen and Kirchhoff further developed the spectroscope by incorporating the Bunsen burner as a source to heat the elements. In 1861, experiments by Kirchhoff and Bunsen demonstrated that each element, when heated to incandescence, gave off a characteristic color of light. When the light was separated into its constituent wavelengths by a prism, each element displayed a unique pattern or emission spectrum. Emission Spectra Complement Absorption Spectra The emission spectrum seemed to be the complement to the mysterious dark lines (Fraunhofer lines) in the sun's spectrum. This meant that it was now possible to identify the chemical composition of distant objects like the sun and other stars. They concluded that the Fraunhofer lines in the solar spectrum were due to the absorption of light by the atoms of various elements in the sun's atmosphere. Hydrogen Spectrum – The Balmer Series In 1885, Johann Jakob Balmer analyzed the hydrogen spectrum and found that hydrogen emitted four bands of light within the visible spectrum. His empirical formula for the visible spectral lines of the hydrogen atom was later found to be a special case of the Rydberg formula, devised by Johannes Rydberg. Johann Jakob Balmer (May 1, 1825 – March 12, 1898) Swiss Mathematician & Honorary Physicist Wavelength (nm) Color 656.2 red 486.1 blue 434.0 blue-violet 410.1 violet Quantum Properties of Light E = nh E – the change in Energy n= 1, 2, 3, … h – (Planck’s constant) h = 6.62610-34 Js Max Karl Ernst Ludwig Planck = frequency (April 23, 1858 – October 4, 1947) German Physicist The Nobel Prize in Physics 1918 for The discovery of energy quanta. The profile of radiation emitted from a black body In 1900, Planck hypothesized that energy was quantized (i.e. energy can be gained or lost only in whole-number multiples of the quantity h.) This hypothesis was later extended by Albert Einstein to include light. Einstein envisioned light as small discrete particles of energy which he called photons. Calculating the Balmer & Lyman Series The four bands of light calculated by Balmer can be simply calculated using the Rydberg equation: 1 1 * R( 2 2 ) n1 n2 Where v = frequency n = the quantum number R = (Rydberg constant) R = 3.29 1015 Hz 1 Hz = 1 s-1 The permitted energy levels of a hydrogen atom. *This equation will be used on page 159. Recall that Frequency and Wavelength are related where frequency times wavelength equals the speed of light. Wavelength (): Distance between two consecutive peaks [unit: nm] Frequency (): Number of waves per second that pass a given point in space [unit: s-1 (Hertz)] = c Where C is the speed of light & C = 2.9979108 m/s Since the speed of light is a constant, as wavelength decreases, then frequency must increase. In 1913, Bohr developed a quantum model for the hydrogen atom. Proposed the Solar System model of the atom where the electron in a hydrogen atom moves around the nucleus only in certain allowed circular orbits. Niels Henrik David Bohr Oct. 7, 1885 – Nov. 18, 1962 Danish Physicist The Nobel Prize in Physics 1922 for the investigation of the structure of atoms and of the radiation emanating from them. https://www.youtube.com/watch?v=-YYBCNQnYNM These orbits then correspond to the energy levels seen in the Balmer series. (p 167) Atomic Spectra Experiment PART A: Hydrogen emission spectrum. PART B: Emission spectrum of other elements. PART C: Tests on elements: Mg, Al, Si, Ca & Zn. PART A: Record Hydrogen line spectrum with a Scanning Spectrophotometer. The hydrogen line spectrum contains only a few discrete wavelengths. In the visible region, there are only four wavelengths. Scanning Spectrophotometer (top view) A hydrogen light source will be viewed using a scanning spectrophotometer. The wavelengths will be calculated for the Balmer and Lyman series and then compared to those generated by the computer attached to the scanning spectrophotometer. Computer Output from a Scanning Spectrophotometer The peaks on the spectrograph correspond to the energy changes of the electrons for the Hydrogen atom. PART B: Emission spectrum of other compounds using The STAR Spectrophotometer. 1. View the line spectrum through the STAR Spectrophotometer - point slit towards the light and view to the right. 2. In the hallway, verify that the scale is lined-up accurately by looking at the fluorescent light. In addition to other lines, you should see a green doublet for mercury at ~570 nm (the scale on the bottom). 3. Measure the line spectrum of the gas tubes set up near chalkboards. Note: The fastest/easiest way to do this is have one partner view the lines and the other write down the observations. 4. Compare your results with NIST literature values. For the fluorescent light compare it to the element mercury. Atomic Spectra of Noble Gases Room 201 & 212 Helium Room 201 & 212 Neon Room 201 only. 212 Students Road Trip! Argon Room 201 only. 212 Students Road Trip! Krypton Room 212 only. 201 Students Road Trip! Xenon The Atomic Spectra will be determined for the Noble Gases by looking at the gas discharge tubes. The Periodic Table A period is a horizontal row of elements in the periodic table. Atomic numbers increase from left to right across the table. There are 7 periods. A group (also called a family) is a vertical column in the periodic table. Elements in a given group have similar electronic configurations for their valence shell electrons, and so they have similar chemical properties. Atomic numbers increase from top to bottom. There are 18 groups. Periodic Properties By looking at the relationship between the chemical reactivity of magnesium, aluminum, silicon calcium & zinc and their positions on the periodic table, we hope to discover periodic trends within the 3rd & 4th period & the alkaline earth metal group (family). Magnesium, 12 Aluminum, 13 Calcium, 20 Zinc, 30 * * * ** Silicon, 14 Some well known periodic trends. Magnesium, Mg (Z=12) Properties: an alkaline earth metal, silvery white, fairly tough, oxidizes slowly in air, burns rapidly in air with a brilliant white flame. Normally magnesium is coated with a layer of oxide, MgO, that protects magnesium from air and water. Available in several forms including chips, granules, powder, rod, foil, sheet, rod, turnings, and ribbon. Magnesium, Mg (Z=12) Properties: a bright white metal that oxidizes slowly in air, burns rapidly in air with a brilliant white flame, 2 Mg(s) + O2(g) → 2 MgO(s) reacts with water at room temperature, Mg(s) + H2O(l) → Mg(OH)2 (aq) and reacts with dilute acids with the liberation of hydrogen gas Mg (s) + HCl(aq) → MgCl2 (aq) + H2(g) Magnesium burns with a bright white flame.* does not appear to react with dilute aqueous alkaline solutions. *Do NOT look directly at the magnesium flame. Aluminum, Al (Z=13) Properties: a silvery-white metal that can be highly polished. It is light, nontoxic (as the metal), a good conductor of heat and electricity, nonmagnetic and nonsparking. Aluminum is ductile and malleable and can be drawn into wire and rolled into sheets. Pure aluminum is soft and lacks strength, but alloys with small amounts of copper, magnesium, silicon, manganese, and other elements have very useful properties. Occurrence: Widely distributed and it is third in abundance on earth after oxygen and silicon. Aluminum, Al (Z=13) Reactions: Aluminum reacts rapidly with the oxygen in air to form aluminum oxide, 4 Al (s) + 3 O2 (l)→ 2 Al2O3(s) Because the surface of aluminium metal is covered with this thin layer of oxide, it is actually protected from further attack by oxygen. So, aluminum metal does not normally react with air or water. If the oxide layer is damaged, the aluminium metal is exposed to attack, even by water. Aluminium metal dissolves in dilute hydrochloric acid 2Al(s) + 6HCl(aq) → 2Al3+(aq) + 6Cl- (aq) + 3H2 (g) Aluminium dissolves in sodium hydroxide with the evolution of hydrogen gas, H2, and the formation of aluminates [Al(OH)4]2Al(s) + 2NaOH(aq) + 6H2O(l) → 2Na+(aq) + 2[Al(OH)4]-(aq) + 3H2(g) Silicon, Si (Z=14) Properties: a semi-metallic element, dark grey with a bluish tinge; amorphous silicon is a dark brown powder. Elemental silicon transmits more than 95% of all wavelengths of infrared and and has been used in lasers to produce coherent light at 456 nm. Occurrence: Silicon is present in the sun and stars and is a principal component of a class of meteorites known as aerolites. Silicon makes up 25.7% of the earth's crust by weight, and is the second most abundant element, exceeded only by oxygen. Silicon, Si (Z=14) Reactions: The surface of lumps of silicon is protected by a very thin layer of silicon dioxide, SiO2. This renders silicon insoluble in water and most acids. Silicon does dissolve in hydrofluoric acid forming fluorosilicic acid, Si(s) + 6 HF (aq) → 2 H2 (g) + H2SiF6 (aq) Silicon dissolves in sodium hydroxide forming sodium silicate, Si (s) + 2 NaOH (aq) + H2O (l) → 2 H2 (g) + Na2SiO3 (aq) and highly complex species containing the anion [SiO4]4Si (s) + 4NaOH (aq) → [SiO4]4- (aq) + 4Na+ (aq) + 2H2 (g) Calcium, Ca (Z=20) Properties: an alkaline earth metal, silver-white, which tarnishes slowly in air and is approximately as hard as tin. Occurrence: Widely found as its carbonate in rocks, chalk, limestone, and marble, and as mixed carbonates in dolomite, gypsum, CaSO4.2H2O, fluorite, CaF2, apatite, Ca5(PO4)3, diopside, CaMg(SiO3)2, and lime feldspar, CaAl2Si2O8. The soluble calcium salts are responsible for the hardness of natural spring water. Calcium, Ca (Z=20) Reactions: highly reactive chemically, oxidizes slowly in air, burns rapidly when heated in air to form calcium oxide, 2 Ca (s) + O2(g) → 2 CaO(s) reacts with water to form calcium hydroxide and hydrogen gas, Ca(s) + 2 H2O(l) → Ca(OH)2(aq) + H2(g) and reacts with dilute acids with the liberation of hydrogen gas, Calcium burns with a light red glow. Ca(s) + HCl(aq) → CaCl2(aq) + H2(g) Zinc, Zn (Z=30) Properties: a transition metal: bluish-white & lustrous. Brittle at ambient temperatures, but malleable at 100-150 oC. Occurrence: available in many forms including dust, foil, granules, powder, pieces, shot, and a mossy form. Flame Test: burns with a light green glow. Zinc, Zn (Z=30) Reactions: When exposed to the atmosphere, zinc reacts with oxygen to form zinc oxide, which reacts with water molecules in the air to form zinc hydroxide. Finally zinc hydroxide reacts with carbon dioxide in the atmosphere to yield a thin, impermeable, tenacious and quite insoluble dull gray layer of zinc carbonate which adheres extremely well to the underlying zinc. 2 Zn(s) + O2(g) → ZnO(s) + H2O(g) → Zn(OH)2 (s) + CO2(g) → 2 ZnCO3(s) when the zinc carbonate surface is scratched, the underlying zinc reacts with water with the liberation of hydrogen gas: Zn (s) + 2 H2O(l) → Ca(OH)2(aq) + H2(g) and reacts with dilute acids with the liberation of hydrogen gas: Zn(s) +2 HCl(aq) → ZnCl2(aq) + H2(g) and in weak basic solutions, it reacts to form zinc hydroxide, a white precipitate: Zn(s) +2 NH4OH (aq) → Zn(OH)2(s) + H2(g) but in stronger basic solutions, this hydroxide is dissolved to form soluble zincates. Zn(OH)2(s) + 2 OH- (aq) → Zn(OH)42-(aq) Checkout – (All items checked out should be returned) STAR Spectroscope Set of Crayons ROYGBV 1 bottle of phenolphthalein 1 flint striker 5 vials with metals: Mg, Al, Si, Ca & Zn 1 piece of sandpaper 6M HCl in a squeeze bottle 6M NaOH in a squeeze bottle In Lab Computerized scanning spectrophotometer – 1 setup in 212 Gas discharge tubes (for viewing by STAR spectroscope) He & Ne on in both rooms. Ar & Kr in 201. Xe in 212. All students View Scanning Spectrophotometer for Part A in Room 212. TAs will let you know when to go to see the demonstration. View Gas Discharge tubes for Part B, located near the chalkboards. Do tests on metals for Part C at your lab bench. Hazards: 6M HCl – strong acid, corrosive (use solid NaHCO3 on spills) 6M NaOH - strong base, corrosive Phenolphthalein – laxative effect if swallowed Bunsen Burner – open flames Waste: Liquid waste - all waste, metals, acid & rinses. For November 17-21 Due: Atomic Spectra & Periodic Properties Handout (Note: Page 159 counts as your calculations page. That is, if calculations are shown on p159, then you do not have to do an additional calculations page. Reactor Trip in Class November 17-21 will take the full 3 hours. Reminder: You must have your student id to go to the reactor. Also: NO backpacks, NO cell phones & NO electronic devices are allowed in the reactor! Read: Radiochemistry (pp 119-136) in Lab packet.