Chemical Bonding
advertisement

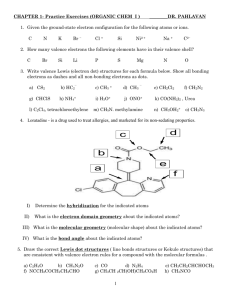
CHEMICAL BONDING Matter is composed of either (1) Metals - Atoms - Metallic Bonding (2) Nonmetals - Molecules - Covalent Bonding (3) Metals and Nonmetals - Ions - Ionic Bonding Chemical bonding involves the valence electrons of atoms 2A-1 (of 15) (2) MATTER COMPOSED OF METALS 1904 ARNOLD SOMMERFELD Proposed that metal atoms release their valence electrons, and share them between large numbers of metal atoms 2A-2 (of 15) METALLIC BOND – The electrostatic attraction of the shared valence electrons to the nuclei of the many bonding metal atoms Metallic bonding forms crystalline networks containing billions of metal ATOMS that are strongly attracted together 2A-3 (of 15) (3) MATTER COMPOSED OF NONMETALS 1916 GILBERT NEWTON LEWIS Proposed that nonmetal atoms share valence electrons to achieve the electron configurations of Noble Gases Diatomic chlorine : : : Cl Cl : : : : : : : : Cl – Cl : 2A-4 (of 15) LEWIS STRUCTURE – A representation of chemical bonding using electron dot notation : : BONDING PAIRS: in red LONE PAIRS: 2A-5 (of 15) in green : : : Cl Cl : COVALENT BOND – The electrostatic attraction of the shared electrons to the nuclei of the bonding nonmetal atoms Covalent bonding forms individual units called MOLECULES that are weakly attracted to each other 2A-6 (of 15) LEWIS STRUCTURES To draw a proper Lewis Structure for a covalently bonded species: 1 – Add up the valence e-s for all of the atoms in the molecule or ion 2 – Draw a skeletal structure by using pairs of electrons to make bonds 3 – Complete octets (or duets for H) for all atoms, outer atoms first, using the remaining valence e-s 4 – If octets are not produced, make the atoms that have octets share more e- pairs with atoms that do not have octets 2A-7 (of 15) Oxygen difluoride, OF2 6 + 7 + 7 = 20 valence e-s F O 2A-8 (of 15) F Nitrogen tribromide, NBr3 5 + 7 + 7 + 7 = 26 valence e-s Br N Br 2A-9 (of 15) Br (1) MATTER COMPOSED OF METALS AND NONMETALS 1904 RICHARD ABEGG Proposed that atoms gain or lose valence electrons to achieve the electron configurations of Noble Gases 2A-10 (of 15) Metal atoms easily lose valence e-s, forming positive ions Al 1s22s22p63s23p1 Al3+ 1s22s22p6 Fe [Ar]4s23d6 Fe2+ [Ar]3d6 Fe3+ [Ar]3d5 Nonmetal atoms gain e-s to their valence shells, forming negative ions O 1s22s22p4 O2- 1s22s22p6 Once these ions are formed, they are stable (or unreactive) 2A-11 (of 15) IONIC BOND – The electrostatic attraction between positive metal ions and negative nonmetal ions Ionic bonding forms crystalline networks containing billions of positive and negative IONS that are strongly attracted together 2A-12 (of 15) Sodium chloride Na . .. Cl : .. .. + Na : Cl .. : - A sodium chloride crystal is a symmetrical array of sodium and chloride ions in a 1:1 ratio EMPIRICAL FORMULA – The simplest whole number ratio of ions of different elements in a compound Empirical Formula: NaCl 2A-13 (of 15) Calcium fluoride Ca . .. F: .. .. 2+ Ca . : F: .. .. F: .. - .. : F: - .. Empirical Formula: CaF2 2A-14 (of 15) REPRESENTING IONIC BONDING WITH ELECTRON DOT NOTATION K3N . K. K. K. + + + . N: . .. K K 2A-15 (of 15) K : N: .. 3- Fluorine, F2 7 + 7 = 14 valence e-s F F SINGLE BOND – One shared pair of e-s between two atoms 2B-1 (of 15) Oxygen, O2 6 + 6 = 12 valence e-s O O DOUBLE BOND – Two shared pairs of e-s between two atoms 2B-2 (of 15) Nitrogen, N2 5 + 5 = 10 valence e-s N N TRIPLE BOND – Three shared pairs of e-s between two atoms 2B-3 (of 15) BOND ORDER – The number of shared pairs of electrons BOND ENERGY – The energy needed to break a bond BOND LENGTH – The distance between the nuclei of the 2 bonding atoms Bond Order Bond Energy (kJ/mol) Bond Length (nm) 2B-4 (of 15) F2 O2 N2 1 154 2 495 0.121 3 941 0.142 0.110 H H Cl Cl I I S S P P Longest Bond Length? I2 biggest atoms Shortest Bond Length? H2 smallest atoms Highest Bond Energy? P2 most bonding electrons Lowest Bond Energy? I2 least bonding electrons, and they are most shielded from the nuclei 2B-5 (of 15) Formaldehyde, CH2O 4 + 1 + 1 + 6 = 12 valence e-s H C H 2B-6 (of 15) O Sulfate, SO426 + 4(6) + 2 = 32 valence e-s 2- O O S O 2B-7 (of 15) O NO35 + 3(6) + 1 = 24 valence e-s - O N O O - ↔ O N O O - ↔ O N O O RESONANCE – When more than one Lewis structure can be drawn for a molecule or ion RESONANCE STRUCTURES – The Lewis structures that can be drawn for the molecule or ion The bonding in the real nitrate ion is an average of its resonance structures The average N-O bond order is (1+1+2) / 3 = 11/3 2B-8 (of 15) 1932 LINUS PAULING Described how atomic orbitals are involved in covalent bonding VALENCE BOND THEORY – Two atoms share electrons by overlapping a valence atomic orbital from each atom, creating a region of space between the nuclei where the electrons reside 2B-9 (of 15) H atom H2 molecule 1s atomic orbital with 1 valence e- H atom 1s atomic orbital with 1 valence e- 2 valence e-s in a MOLECULAR ORBITAL The attraction of the e-s in the molecular orbital to the 2 nuclei bonds the atoms together 2B-10 (of 15) ELECTRONEGATIVITY – A property developed by Pauling, measuring the attraction of an atom for shared electrons Metals – Low EN’s (the most active metals having the lowest EN’s) Nonmetals – High EN’s (the most active nonmetals have the highest EN’s) Atom with the highest EN? Atom with the lowest EN? F Cs (4.0) 2B-11 (of 15) (0.7) EN differences between atoms indicates their type of bonding EN Difference Bonding 0 Small (0.1 – 1.6) Large (1.7 – 3.3) Nonpolar Covalent Polar Covalent Ionic 2B-12 (of 15) 2 atoms with the same EN’s have an EN difference of 0 N–N (EN of N = 3.0) NONPOLAR COVALENT BOND – A bond between 2 atoms in which the electrons are shared evenly 2B-13 (of 15) 2 atoms with close EN’s have an EN difference that is small H – Br (EN of H = 2.1, EN of Br = 2.8) Dipole Moment Arrow POLAR COVALENT BOND – A bond between 2 atoms in which the electrons are shared unevenly 2B-14 (of 15) 2 atoms with extreme EN’s have an EN difference that is large Na – Cl (EN of Na = 0.8, EN of Cl = 3.0) IONIC BOND – A bond between 2 atoms in which the electrons are transferred, creating ions 2B-15 (of 15) FORMAL CHARGE While atoms that covalently bond are not charged, they can be given charges based upon where the bonding electrons are assigned FORMAL CHARGE – The charge given to an atom assuming one electron in each bond is assigned to that atom 2C-1 (of 11) F F. 0 S . F S. 0 . F 0 S naturally has 6 valence e-s , and now 6 0 F naturally has 7 valence e-s , and now 7 0 Quick way to determine formal charge: (natural number of valence e-s – 1 e- per bond – each lone pair e-) S: F: 2C-2 (of 11) 6–2–4= 0 7–1–6= 0 C O C O -1 +1 C: 4 – 3 – 2 = -1 O: 6 – 3 – 2 = +1 Formal charges are used to determine the validity of a Lewis structure the most accurate Lewis structures are those with atoms that have formal charges as close to 0 as possible 2C-3 (of 11) thiocyanate, SCN6 + 4 + 5 + 1 = 16 valence e-s S S: C: N: C N - 6–2–4= 0 4–4–0= 0 5 – 2 – 4 = -1 2C-4 (of 11) ↔ S C N - S: 6 – 3 – 2 = +1 C: 4 – 4 – 0 = 0 N: 5 – 1 – 6 = -2 ↔ S C N - S: 6 – 1 – 6 = -1 C: 4 – 4 – 0 = 0 N: 5 – 3 – 2 = 0 thiocyanate, SCN6 + 4 + 5 + 1 = 16 valence e-s S S: C: N: C N - 6–2–4= 0 4–4–0= 0 5 – 2 – 4 = -1 ↔ S C N - S: 6 – 3 – 2 = +1 C: 4 – 4 – 0 = 0 N: 5 – 1 – 6 = -2 ↔ S C N S: 6 – 1 – 6 = -1 C: 4 – 4 – 0 = 0 N: 5 – 3 – 2 = 0 The best Lewis structures have (1) formal charges for the most atoms as close to 0 as possible (2) negative formal charges go on the atom with the greatest EN 2C-5 (of 11) - COVALENT COMPOUNDS THAT DO NOT OBEY THE OCTET RULE (1) Molecules with hypovalent central atoms (atoms with less than 4 valence electrons) Covalent compounds with B and Be BeH2 2 + 1 + 1 = 4 valence e-s H Be 2C-6 (of 11) H BF3 3 + 7 + 7 + 7 = 24 valence e-s F B F F F B F 2C-7 (of 11) F NO! F is too electronegative to share more than 1 pair of e-s (2) Molecules with hypervalent central atoms (atoms that have empty d orbitals in their outer shell) Nonmetal atoms in the 3rd, 4th, 5th, or 6th Periods PF5 5 + 5(7) = 40 valence e-s 3s F F F P F 2C-8 (of 11) F ↑↓ ___ 3p ↑ ↑ ↑ ___ ___ ___ 3d ___ ___ ___ ___ ___ P can make 5 bonds using empty d orbitals in its outer shell ClF3 7 + 3(7) = 28 valence e-s F F Cl F F F Cl 2C-9 (of 11) F Only 26 valence e-s Sulfate, SO426 + 4(6) + 2 = 32 valence e-s 2- O O S S: O 6 – 4 – 0 = +2 O: 6 – 1 – 6 = -1 O Experimental data shows the S-O bonds are stronger than single bonds Reducing the formal charge on atoms that can exceed the octet rule can produce a more accurate Lewis structure S must make 2 double bonds to reduce its formal charge to 0 2C-10 (of 11) Sulfate, SO426 + 4(6) + 2 = 32 valence e-s 2- O O S O O + 5 other resonance structures S: 6 – 6 – 0 = 0 O: 6 – 1 – 6 = -1 O: 6 – 2 – 4 = 0 Experimental data shows the S-O bonds are stronger than single bonds Reducing the formal charge on atoms that can exceed the octet rule can produce a more accurate Lewis structure S must make 2 double bonds to reduce its formal charge to 0 2C-11 (of 11) MOLECULAR SHAPE VSEPR THEORY (Valence Shell Electron Pair Repulsion) – All atoms and lone pairs attached to a central atom will spread out as far as possible to minimize repulsion A Lewis structure must be drawn to use the VSEPR Theory 2D-1 (of 15) CO2 4 + 6 + 6 = 16 valence e-s O C O STERIC NUMBER (SN) – The sum of the bonded atoms and lone pairs on a central atom The steric number of carbon is 2 (SN = 2): 2 bonded atoms and 0 lone pairs Linear Bond angle is 180° 2D-2 (of 15) O C O BH3 3 + 1 + 1 + 1 = 6 valence e-s H B H H SN = 3 3 bonded atoms and 0 lone pairs H Trigonal Planar Bond angle is 120° 2D-3 (of 15) B H H SO2 6 + 6 + 6 = 18 valence e-s O S O SN = 3 2 bonded atoms and 1 lone pairs Bent Bond angle is 120° 2D-4 (of 15) O S O CH4 H H C H H SN = 4 4 bonded atoms and no lone pairs H Tetrahedral Bond angle is 109.5° 2D-5 (of 15) C H H H NH3 H N H H SN = 4 3 bonded atoms and 1 lone pairs Trigonal Pyramidal Bond angle is 108° 2D-6 (of 15) N H H H H2O .. H–O: H SN = 4 2 bonded atoms and 2 lone pairs Bent Bond angle is 105° 2D-7 (of 15) O H H PF5 F F P F F SN = 5 5 bonded atoms and no lone pairs F Trigonal Bipyramidal 3 Equatorial F’s in a plane, 120° apart 2 Axial F’s 180° apart, 90° from the plane 2D-8 (of 15) F F F P F F SF4 :F: F S F :F: SN = 5 4 bonded atoms and 1 lone pair ← 2 close 90º interactions 3 close 90º interactions → ← most stable configuration e- pair in equatorial position 2D-9 (of 15) e- pair in axial position SF4 :F: F S :F: F SN = 5 4 bonded atoms and 1 lone pair See-Saw e- pairs always go in equatorial positions to minimize repulsion 2D-10 (of 15) F F F P F ClF3 F Cl F F SN = 5 3 bonded atoms and 2 lone pairs F T-Shape Cl F 2D-11 (of 15) F XeF2 F Xe F SN = 5 2 bonded atoms and 3 lone pairs F Linear Xe F 2D-12 (of 15) SF6 F F F S F F SN = 6 6 bonded atoms and no lone pairs F F Octahedral 90º and 180º F F S F 2D-13 (of 15) F F IF5 F F F I F F SN = 6 5 bonded atoms and 1 lone pair F Square Pyramidal 2D-14 (of 15) F F I F F XeF4 F F Xe F SN = 6 4 bonded atoms and 2 lone pairs F Square Planar 2D-15 (of 15) F F Xe F F MOLECULAR POLARITY A BOND is polar if it has a positive end and a negative end A MOLECULE is polar if it has a positive end and a negative end To determine if a molecule is polar or nonpolar: 1) Draw the correct Lewis structure 2) Draw its correct shape 3) Use EN’s to determine if the BONDS in the molecule are polar or nonpolar 4) For the polar bonds, label the positive and negative ends with δ+ and δ5) If a line can be drawn separating all δ+’s from all δ-’s, the molecule is polar, if not its nonpolar 2E-1 (of 13) .. H–O: H δ+ H δO δ- H δ+ 2E-2 (of 13) EN’s: O = 3.5, H = 2.1 3.5 – 2.1 = 1.4 the O-H BONDS are polar All of the δ+’s can be separated from all of the δ-’s, the H2O MOLECULE is polar H N H H EN’s: N = 3.0, H = 2.1 δδ- N δδ+ H H δ+ 2E-3 (of 13) 3.0 – 2.1 = 0.9 the N-H BONDS are polar H δ+ All of the δ+’s can be separated from all of the δ-’s, the NH3 MOLECULE is polar F F C F F δF δ+ δ+ C δ+ δ- F δ+ F δF δ- 2E-4 (of 13) EN’s: C = 2.5, F = 4.0 4.0 – 2.5 = 1.5 the C-F BONDS are polar All of the δ+’s cannot be separated from all of the δ-’s, the CF4 MOLECULE is nonpolar A more exact way to determine if a molecule is polar or nonpolar: 1) Draw the correct Lewis structure 2) Draw its correct shape 3) Use EN’s to determine if the BONDS in the molecule are polar or nonpolar 4) For the polar bonds, draw a DIPOLE MOMENT ARROW pointing toward the negative end of the bond 5) If the dipole moments are symmetrical the molecule is NONPOLAR 2E-5 (of 13) Dipole moments of equal magnitude are symmetrical if: 1) there are 2 dipole moments that are linear Y 2E-6 (of 13) X Y Dipole moments of equal magnitude are symmetrical if: 2) there are 3 dipole moments that are trigonal planar Y X Y 2E-7 (of 13) Y Dipole moments of equal magnitude are symmetrical if: 3) there are 4 dipole moments that are tetrahedral Y Y Y 2E-8 (of 13) X Y O O C C 2E-9 (of 13) O O Symmetrical dipole moments the CO2 MOLECULE is nonpolar .. H–O: H O H H 2E-10 (of 13) Assymmetrical dipole moments the H2O molecule is POLAR H N H H N H H 2E-11 (of 13) H Assymmetrical dipole moments the NH3 molecule is POLAR F F C F F F C F F 2E-12 (of 13) Symmetrical dipole moments the CF4 molecule is NONPOLAR F Cl F C F F Cl C F F 2E-13 (of 13) F Assymmetrical dipole moments because the C-Cl dipole moment is smaller than the C-F dipole moments the CClF3 molecule is POLAR