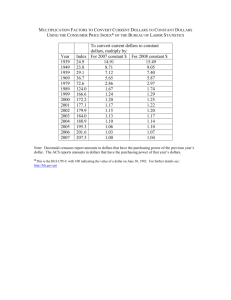
Unit 02 Quant Chem
advertisement

Measurements and Density By the end of today’s lesson, be able to: 1.) Calculate mass, volume, and density 2.) Measure to one digit of uncertainty A measurement of “compactness”: how close the atoms are to one another Mass Volume Density = Mass Volume Mass = measurement of the amount of matter (atoms and molecules) Volume = the amount of space an object occupies Calculate the mass of a rectangular prism WITHOUT putting it on the balance! ◦ HINT: Make some measurements and calculate the volume. Color of Block Color of Tape Density (g/cm3) Paper-white Green 0.541 Black white 0.967 Cloudy-white Orange 0.910 Clear Red 1.17 Gray Yellow 1.42 Number 15.2 Measurement 15.2 mL Apple + Shadow Width = 14 cm , 0cm , 10cm , 20cm , 30cm Apple + Shadow Width = 14.4 cm , 0cm , 10cm , 20cm , 30cm Measure at the bottom of the meniscus Calculate the mass of a rectangular prism WITHOUT putting it on the balance! ◦ HINT: Make some measurements and calculate the volume. Color of Block Color of Tape Density (g/cm3) Paper-white Green 0.541 Black white 0.967 Cloudy-white Orange 0.910 Clear Red 1.17 Gray Yellow 1.42 Which mass was closer? What is the percent error of your two calculations? (0% error is perfect; there is NO error) To determine the number of sig figs and use them for rounding To show set up for unit conversion (factorlabel method) 1) 2) 3) All nonzero numbers are significant “Placeholder” zeros are not significant. Measured zeros are significant (Atlantic/Pacific Rule) In scientific notation, ALL numbers written before the “x 10exp” are significant 1.230 x 104 90.210 0.0090210 90210. 90210 1) 2) Multiplication/Division: Report the same number of sig figs as the value with the least. 90210 x 90.210 = 8137844.1 Addition/Subtraction: Report the same number of decimal places as the value with the least. 90210 + 90.210 = 90300.21 A unit equation is a simple statement of two equivalent quantities. ◦ 1 km = 1000 m ◦ 1 cm = 0.01 m ◦ 100 cm = 1m 100 cm = 1 m “centi” (c) 1000 mm = 1m “milli” (m) 1 km = 1000 m “kilo” (k) Prefixes can be applied to ANY unit, like grams or Liters A unit conversion factor, or unit factor, is a ratio of two equivalent options. For the unit equation 60 minutes = 1 hour, we can write two unit factors: 1 hour 60 minutes or 60 minutes 1 hour =1 Start with your given value in fraction form “7.39 dollars” 7.39 dollars ____________ 1 Treat units like variables so that they will cancel. 7.39 dollars 1 quarter ____________ x _____________ 1 0.25 dollars 7.39 dollars dollars ____________ x _____________ 1 quarter If you have $7.39, how much is this amount in quarters? A candy bar is approximately 220 grams. How many ounces is this? If there are 24.6 blips to every zazzle and there are 5.7 pinches to every blob and 3 blobs in a zazzle, how many blips are in 0.95 pinches? Density of an object is 4.90 g/mL what is the density of this object in pounds per Liter? A cube is measured to have a volume of 13,506m3, what is this volume in km3 ?