Calculating Percent Error
advertisement

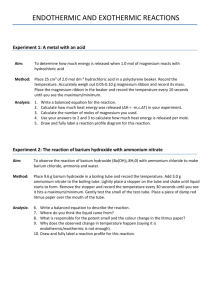
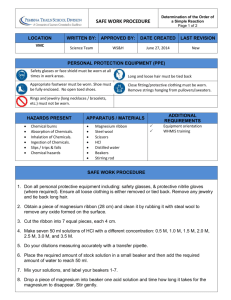
Because nobody gets it perfectly right When an experiment is done, it is pretty much certain that the answer is not going to be exactly correct. Many variables and actions go into an experiment. A change or a misstep in any one of them can result in an answer that is a bit different from the actual value. Actions such as mismeasurement, lack of purity of reagents, and poor lab technique can all result in error. There are several ways that error can be described. SYSTEMATIC ERROR v. RANDOM ERROR POSITIVE PERCENT ERROR v. NEGATIVE PERCENT ERROR ◦ Systematic Error is when the error is built into the lab and there is nothing the experimenter can do about it. ◦ Example: If you were given a sample of Iron to determine its density and the sample wasn’t pure iron, it would constantly and consistently throw off your values. Random Error is when the lab mistake can happen differently from lab to lab. Example: Let’s say you are making a solution and it needs to be stirred and then put aside. There can be solution lost by stirring and splashing material out of the beaker. Some water might evaporate out at different rates as the solution is put aside. Positive Error is when the value the experimenter gets is greater than the actual value. The actual value is considered the ‘right answer’. Example: If you are determining the concentration of a solution and you determine it to be 2.5 M and it is only 2.2 M then you will record a positive error. Negative Error is when the value the experimenter gets is less than the actual value. In Chemistry, it is very common to get negative percent errors in your work. Example: If you are determining the concentration of a solution and you determine it to be 1.5 M and it is 2.2 M then you will record a negative error. We use the following formula. ◦ O Observed Result (what you got) ◦ A Actual Result (what you should get) ◦(( O – A) / A ) x 100 % ◦ You will use this often in Lab. A Lab group was working in a Lab to determine the percent of Magnesium in a sample of Magnesium Oxide. They determined it to be 58.3 % They looked up the actual value and found it to be 60.3 % What is their percent error ? Let’s start with the formula (( O – A) / A ) x 100 % Substituting in gives us the following: ◦ ((58.3 – 60.3) / 60.3 ) x 100 % ◦ ( - 2 / 60.3) - .00331 ◦ - .00331 x 100 % = - 3.31 % A different group doing the same lab got a value of 68.6 % Mg. What is their percent error ? Using the same formula (( O – A) / A ) x 100 % Substituting in gives us: ◦ ((68.6 – 60.3 ) / 60.3 ) x 100 % ◦ (8.3 / 60.3) x 100 % .1376 ◦ .1376 x 100 % = 13.76 % The mass of the Magnesium Oxide sample could have been measured incorrectly. Product could have been lost while performing the steps of the lab. There could have been impurities in the sample. The scale could be miscalibrated making the mass readings consistently off. For your labs, you will have to complete an error page. It will include percent error if it is a numerical lab. It will always include “Sources of Error” Make sure that you don’t include errors that didn’t happen. ◦ Don’t say you dropped your beaker if you didn’t You can include possible systematic errors that might be present. ◦ An impure sample ◦ Miscalibrated lab equipment The class website has more detail if needed.