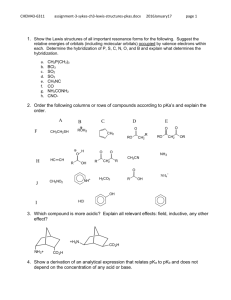
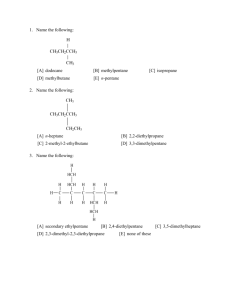
Answers - Chemistry Courses: About
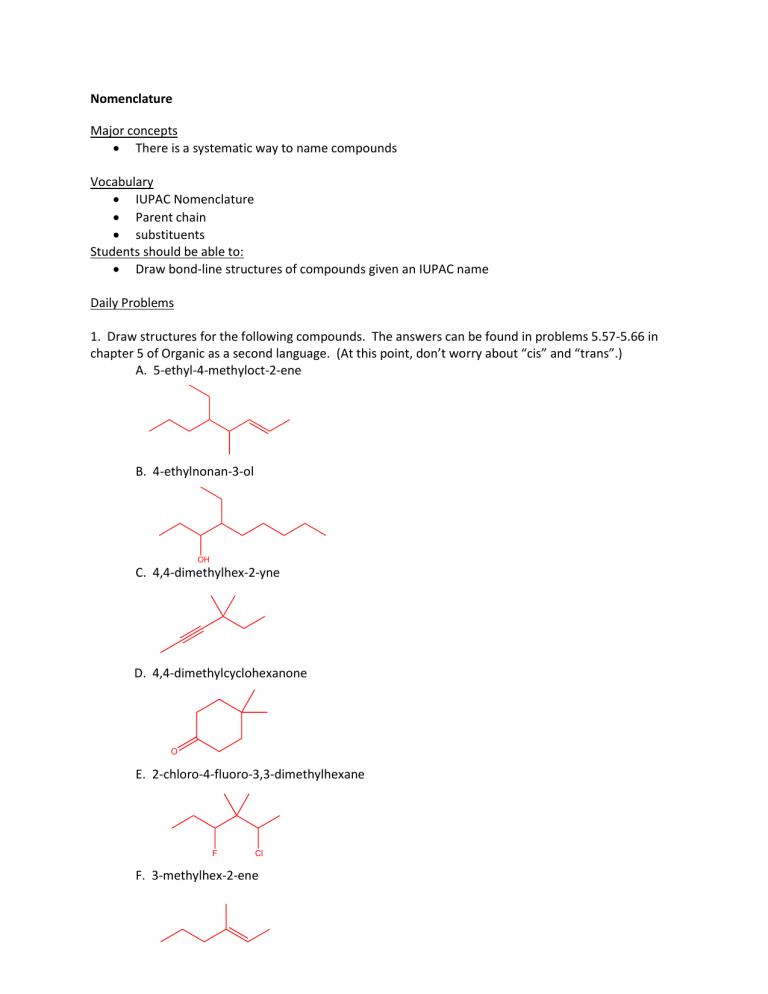
Nomenclature
Major concepts
There is a systematic way to name compounds
Vocabulary
IUPAC Nomenclature
Parent chain
substituents
Students should be able to:
Draw bond-line structures of compounds given an IUPAC name
Daily Problems
1. Draw structures for the following compounds. The answers can be found in problems 5.57-5.66 in chapter 5 of Organic as a second language. (At this point, don’t worry about “cis” and “trans”.)
A. 5-ethyl-4-methyloct-2-ene
B. 4-ethylnonan-3-ol
OH
C. 4,4-dimethylhex-2-yne
D. 4,4-dimethylcyclohexanone
O
E. 2-chloro-4-fluoro-3,3-dimethylhexane
F Cl
F. 3-methylhex-2-ene
G. 2-ethylpentanamine
NH
2
H. 2-propylpentanoic acid
O
O
I. oct-2-en-4-ol
OH
OH
J. 5-chloro-6-fluoro-5,6-dimethyloct-2-ene
Cl
F
2. Draw bond-line structures for these compounds. Draw your structures with appropriate angles for all bonds, especially alkynes.
A. 3-chloro-4-methylnonanal
H Cl
B. 4,5-diiodopent-1-yne
I
I
C. 2-aminocyclopropanol
H
2
N
D. hex-3-yne
E. hept-4-en-2-yne
OH
F. 2,2,3,4-tetramethylpentane
Cumulative problems
3. For each of the following reactions, something was added to, or removed from, the starting material.
What has been added or removed?
A. 4-methylpent-2-ene 3-bromo-2-methylpentane
Br
HBr was added
B. 2-methylcyclopentanol
OH
1-methylcyclopentene
H
2
O was removed
4. Categorize these compounds in one of the following three options: Lewis acid, Lewis base, or
Contains both Lewis acidic and basic centers.
A. 3-methylhex-5-enal
H
LA
O
LB
B. but-3-ynoic acid
O
LB
LA OH
LB
C. 3,3,4-triethylcyclooctanone
O
LB
LA
D. cyclobutanamine
LB
NH
2
E. pentane-1,3-diol
LB
OH
HO
LB
F. hex-1-en-4-yne
G. 3-aminoheptanoic acid
LB
NH
2
O
LB
LB
LA
LB
O
LA
OH
LB
H. 2-hydroxycyclopent-3-enone
Extension problems
OH
LB
5. Some of these functional groups have too many hydrogen atoms, which give them a formal charge which is not zero. Indicate which functional groups have an extra hydrogen atom, and then draw a “+” sign beside them.
+ Protonated ketone
Protonated ether
+ alcohol ketone alcohol epoxide alcohol
+
Protonated alcohol ether ketone
+
Protonated ether
When a functional group has an extra hydrogen atom, we say that it is “protonated.” We can then say that a functional group is “protonated”—for example, we may see a “protonated ketone” or a
“protonated ether.” Name all the functional groups above, calling them “protonated” if necessary.