Enthalpy, Chemical Reactions, Hess' Law
advertisement

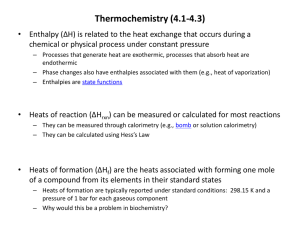
• • • • • Physical Science Walk-In Tutoring Center Physical Sciences Building Room 122 Tuesdays 5:30-8:30 pm Wednesdays 5:30-8:30 pm Feel like you’re trapped in a flask or like Newton’s apple has just hit you on the head? Chemistry, Physics, and Earth Science tutors are available to help! FREE, no-appointment, walk-in tutoring is available for: CHEM 100, 112, & 226; PHYS 100, 103/104, & 204; ESCI 100, GEOL 120, & METR 110 Funding is provided by the Provost. Heating Curves Measuring DH DH and Reactions • Qualitative- phase changes • Quantitative- Heat of fusion/vaporization • Constant Pressure Calorimetry (“coffee cup”) • Constant Volume Calorimetry (bomb) • Hess’ Law (this week’s lab) • Standard Heats of Formation Thermal Energy and Phase Changes Which line segment represents a point at which you would use the heat capacity of the liquid to calculate heat exchange? 1. 2. 3. 4. 5. A->B B->C D->E F->G H->J 48% 16% 16% 13% 7% 1 2 3 4 5 Enthalpy Changes and Chemical Reactions DH = energy needed to break bonds – energy released forming bonds Calorimetry is used to measure enthalpy changes Constant Pressure gives DH Constant Volume gives DE Hess’ Law- The Rules Enthalpy is a state function. If a reaction can be written as the sum of two or more reactions, the DH for the net reaction is the sum of the DH's for the individual steps. If a reaction is reversed, the sign of DH is reversed. If the coefficients of the reactants/products are multiplied by a number, then the DH must be multiplied by that number, as well. Hess’ Law If you can add the reactions, you can add the DH’s. Hess’s Law Hess’ Law- Lab This Week Mg(s) + ½ O2(g) MgO(s) DH = ??? Constant Pressure Calorimetry (“Coffee Cup”) Constant Volume Calorimetry (“Bomb”) N2H4 + 3 O2 2 NO2 + 2 H2O Ereleased = Eabsorbed by water + Eabsorbed by calorimeter Ewater = Ecalorimeter = Total E = DH = energy/moles = 0.500 g N2H4 600 g water 420 J/oC Calculating Reaction Enthalpies Use values that have been determined from experiment Two types of data that can be used Standard enthalpies of formation Bond enthalpies Standard Enthalpy of Formation Standard conditions: pure form, 1 bar pressure, usually at 298K (25°C) Heat of formation for an element in its most stable form is zero Standard heat of formation is given in Joules per mole Results in fractional coefficients on occasion D H D H D H r xn f f prod ru ea ct ct s ants Table 5-2, p. 195 Using Standard Enthalpies of Formation What is the DHrxn for the detonation of nitroglycerin? How much energy is released when 10g is detonated? 1 2 C H ( NO ) ( l ) 3 N ( g ) ( g ) 6 CO ( g ) 5 H O ( g ) 3 5 3 3 2 O 2 2 2 2 Bond Enthalpy Remember that bond energy is the amount of energy required to break a bond in a gas phase molecule Can only use bond enthalpies for reactions in which everything is in the gas phase When calculating DH using bond enthalpies, assume all bonds are broken in the reactants (DH= +) and formed in the products (DH= -) D H broke form rxn Bond Enthalpy D H brok fo rxn Formation of water 2 H O 2 H O 2 2 2 Chemical Reactions and Enthalpy Change C3H8(g) + 5 O2(g) 3 CO2(g) + 4 H2O(g) What does this mean? How much energy is released by a 468-g tank of propane? DH = -2045 kJ