PBPPharmCalc5_MEQ
advertisement

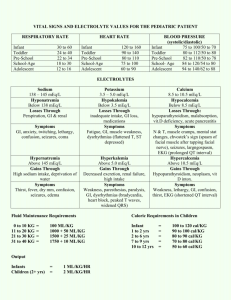
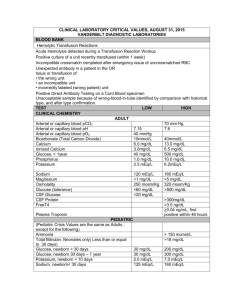
Pharmaceutical Calculations: Electrolyte Solutions – Milliequivalents, Millimoles, Milliosmols Danielle DelVillano, Pharm.D. Objectives • Calculate the milliequivalent weight from an atomic or formula weight • Convert between milligrams and milliequivalents • Calculate problems involving milliequivalents, millimoles, and milliosmols Milliequivalents • A chemical unit: mEq • Related to the total number of ionic charges in solution, and takes note of valence of ions 1 mEq = atomic weight (mg) valence # 1 Eq = atomic weight (g) valence # Equivalent Values Molecular Weights To Know • • • • • • H O Na Cl K Ca • • • • NaCl KCl CaCl2 Dextrose Equations mEq = mg substance * Valence Atomic Weight mg = mEq substance * Atomic weight Valence # mg/mL = mEq/mL substance * Atomic weight Valence # Problem 1 • What is the concentration, in mg/mL, of a solution containing 2 mEq of potassium chloride (KCl) per mL? mg/mL = 2 mEq/mL * 74.5 1 mg/mL = 149 mg/mL Problem 2 • What is the percent (w/v) concentration of a solution containing 100 mEq of ammonium chloride per liter? (MW = 53.5) 1 mEq = 53.5 mg = 53.5 mg 1 100 mEq = x mEq x = 10 mEq 1000 mL 100 mL 1 mEq = 53.5 mg 10 mEq x mg x = 535 mg = 0.535 g per 100 mL or 0.535% Problem 3 • A solution contains 10 mg/100 mL Ca++ ions. Express this concentration in terms of mEq/L 10 mg/ 100 mL * 10/10 = 100 mg/1000 mL mEq/L = 100 mg/L * 2 40 mg Answer = 5 mEq/L Problem 4 • How many mEq of KCl are represented in a 15 mL dose of 10% w/v KCl elixir? 10% = 10g/100 mL 10 g = x g 100 mL 15 mL x = 1.5 g = 1500 mg mEq = 1500 mg * 1 74.5 mg Answer = 20.13 mEq Millimoles and Micromoles • Mole - MW of a substance in grams • Millimole - MW of a substance in mg • Micromile - MW of a substance in mcg • Measure representing the combining power of a species – Monovalent species: mEq = mmol Keep In Mind • Eq wt of a substance = MW / valance • Moles of a substance = MW Problem 5 • How many millimoles of monobasic sodium phosphate (MW 138) are present in 100 g of a substance? 1 mmol = 138 mg x mmol 100000 mg x = 724.6 mmol Osmolarity • Osmotic pressure is proportional to the total number of particles in a solution • Measured in mOsmol • Example – 1 mmol dextrose = 1 mOsmol total particles – 1 mmol NaCl = 2 mOsmol total particles – 1 mmol CaCl2 = 3 mOsmol total particles – 1 mmol Na3C6H5O7 = 4 mOsmol total particles Equation and Definitions • mOsmol/L = g/L substance * # species * 1000 MW • Osmolarity – mOsmol/L solution • Osmolality – mOsmol/kg of solvent Problem 6 • What is the ideal osmolarity of a 0.9% sodium chloride injection? 0.9g/100 mL * 10/10 = 9g/1000mL mOsmol/L = 9g/1L * 2 species * 1000 58.5 g Answer = 308 mOsmol/L Problem 7 • A solution contains 10 mg% of Ca++ ions. How many milliosmols are represented in 1 liter of the solution? 10 mg/100 mL *10/10 = 100 mg/1000mL mOsmol/L = 0.1 g/L * 1 species 40 g Answer = 2.5 mOsmol * 1000 Clinical Considerations of Water and Electrolyte Balance • Total body water for an adult male is 55-65% body weight – Females about 10% lower – Newborn infants 75% • Daily requirement equations – 1500 mL per square meter of BSA – 32 mL/kg for adults – 100-150 mL/kg for infants Equation • Plasma Osmolality (mOsmol/kg) = 2(Na + K) plasma + BUN + Glucose 2.8 18 Na and K measured in mEq/L BUN and glucose measured in mg/100mL (or mg/dL) Problem 8 • Calculate the estimated daily water requirement for a healthy adult with a body surface area of 1.8 m2. 1 m2 = 1500 mL 1.8 m2 x mL x = 2700 mL Problem 9 • Estimate the plasma osmolality from the following data: sodium 135 mEq/L, potassium 4.5 mEq/L, BUN 14 mg/dL, glucose 90 mg/dL mOslmol/kg = 2(135+4.5) Answer 298 mOsmol/kg + 14 + 90 2.8 18 Questions Reference • Ansel, H. C. (2009) Phamaceutical Calculations (13th Ed.). Philadelphia:Lippincott Williams & Wilkins, and Wolters Kluwer Publishers Chapter 12 Page 197 • Calculate he mEq of sodium, potassium, and chloride, the millimoles of anhydrous dextrose, and the osmolarity of the following paerenteral fluid. – Dextrose, anhydrous – NaCl – KCl – Water for injectoin ad 50 g 4.5 g 1.49 g 1000 mL Chapter 12 Page 197 Chapter 12 Page 191 • How many mEq of Na+ would be contained in a 30 mL dose of the following solution? – Disodium hydrogen phosphate – Sodium biphosphate – Purified water ad 18 g 48 g 100 mL (Disodium hydrogen phosphate MW 268) (Sodium biphosphate MW 138) Chapter 12 Page 191 Chapter 12 Problem 3 • A 10-mL ampule of potassium chloride contains 2.98 g of potassium chloride (KCl). What is the concentration of the solution in terms of milliequivalents per milliliter? Chapter 12 Problem 40 • A patient has a sodium deficit of 168 mEq. How many milliliters of isotonic sodium chloride solution (0.9% w/v) should be administered to replace the deficit? Chapter 12 Problem 56 • What is the osmolarity of an 8.4% w/v solution of sodium bicarbonate? Chapter 12 Problem 58 • How many (a) millimoles, (b) milliequivalents, and (c) milliosmoles of calcium chloride (CaCl2⋅2H2O—m.w. 147) are represented in 147 mL of a 10% w/v calcium chloride solution? Equations; Relating mOsmol, mmol and mEq • • • • • mEq = (mg substance * valance) / MW mEq = MW (mg) / valance mmol = MW (mg) mOsmol = (Weight(g) / MW(g)) * species * 1000 mOsmol/kg = 2(Na + K) + (BUN/2.8) + (glucose/18) • mOsmol = mmol * # species • mOsmol = (mEq * # species) valence