Chemistry 100(02) Fall 2013
Instructor: Dr. Upali Siriwardane
e-mail: upali@coes.latech.edu
Office: CTH 311
Phone 257-4941
Office Hours: M,W, 8:00-9:30 & 11:30-12:30 a.m
Tu,Th,F 8:00 - 10:00 a.m. Or by appointment
Test Dates:
September 30,
2013 (Test 1): Chapter 1 & 2
October 21,
2013 (Test 2): Chapter 3 & 4
November 13, 2013 (Test 3) Chapter 5 & 6
November 14, 2013 (Make-up test) comprehensive:
Chapters 1-6 9:30-10:45:15 AM, CTH 328
CHEM 100, Fall 2013 LA TECH
3-1
Text Book & Resources
REQUIRED :
Textbook: Principles of Chemistry: A Molecular Approach,
2nd Edition-Nivaldo J. Tro - Pearson Prentice Hall and also
purchase the Mastering Chemistry
Group Homework, Slides and Exam review guides and
sample exam questions are available online:
http://moodle.latech.edu/ and follow the course information
links.
OPTIONAL :
Study Guide: Chemistry: A Molecular Approach, 2nd EditionNivaldo J. Tro 2nd Edition
Student Solutions Manual: Chemistry: A Molecular
Approach, 2nd Edition-Nivaldo J. Tro 2nd
CHEM 100, Fall 2013 LA TECH
3-2
Chapter 3. Molecules, Compounds, and Chemical
Equations
3.1 Hydrogen, Oxygen, and Water…………………………….
3.2 Chemical Bonds……………………………………………
3.3 Representing Compounds: Chemical Formulas and Molecular Models..
3.4 An Atomic-Level View of Elements and Compounds……………..
3.5 Ionic Compounds: Formulas and Names……………………
3.6 Molecular Compounds: Formulas and Names………………………
3.7 Formula Mass and the Mole Concept for Compounds…………
3.8 Composition of Compounds……………………………..
3.9 Determining a Chemical Formula from Experimental Data………
3.10 Writing and Balancing Chemical Equations……………………
3.11 Organic Compounds……………………….
CHEM 100, Fall 2013 LA TECH
3-3
78
80
82
84
87
93
97
100
105
110
114
Chapter 3. KEY CONCEPTS
• Writing Molecular and Empirical
Formulas (3.3)
• Classifying Substances as Atomic
Elements, Molecular Elements,
Molecular Compounds, or Ionic
Compounds (3.4)
• Writing Formulas for Ionic
Compounds (3.5)
• Naming Simple Ionic Compounds
(3.5)
• Naming Ionic Compounds Containing
Polyatomic Ions (3.5)
• Naming Molecular Compounds (3.6)
• Naming Molecular Compounds (3.6)
• Naming Acids (3.6)
• Calculating Formula Mass (3.7)
• Using Formula Mass to Count
Molecules by Weighing (3.7)
• Calculating Mass Percent
Composition (3.8)
CHEM 100, Fall 2013 LA TECH
• Using Mass Percent Composition
as a Conversion Factor (3.8)
• Using Chemical Formulas as
Conversion Factors (3.8)
• Obtaining an Empirical Formula
from Experimental Data (3.9)
• Calculating a Molecular Formula
from an Empirical Formula and
Molar Mass (3.9)
• Obtaining an Empirical Formula
from Combustion Analysis (3.9)
• Balancing Chemical Equations
(3.10)
3-4
Elements and Compounds Groupings
•
–
•
–
•
–
•
–
•
–
•
–
Atomic elements :
elements whose particles are single atoms (He)
Molecular elements:
elements whose particles are multi-atom molecules (O2)
Molecular compounds:
compounds whose particles are molecules made of only
nonmetals (H2O)
Ionic compounds:
compounds whose particles are cations and anions (NaCl)
Metallic elements
elements whose particles are made up of metal atoms
(Cu)
Metallic compounds (alloy)
compounds whose particles are mixture of metal atoms
(Cu-Zn)
CHEM 100, Fall 2013 LA TECH
3-5
Ionic vs. Molecular Compounds
The compound propane contains
individual C3H8 molecules.
The sodium chloride molecule, NaCl,
is composed of an array of
Na+ ions and Cl– ions.
The platinum, Pt, metal
is composed of an array of
Pt atoms.
CHEM 100, Fall 2013 LA TECH
3-6
Intramolecular Chemical Bonding Types
Ionic:
Complete transfer of 1 or more electrons
from one atom to another, usually between
a metal and a nonmetal element
Covalent:
The sharing of valence electrons shared
between nonmetal elements
Metallic:
The communal sharing of electrons
between metals
*Note: Most molecular bonds are actually
somewhere in between covalent and ionic types.
CHEM 100, Fall 2013 LA TECH
3-7
Types of Compounds
A) Molecular or Covalent Compounds:
non-metal + non-metal
nonmetal oxide or halides: SO2
Organic compounds: C3H8
B) Ionic compounds:
Metal + non-metal:
a) Type I ionic compound
(fixed charge) NaCl
b) Type II ionic compound
FeCl2 and FeCl3, SnCl2 and SnCl4
CHEM 100, Fall 2013 LA TECH
3-8
Formula of a Compound
Formula are used to represent
elements and compound.
For molecular compounds, formula
tell how many of each kind of
atom are in a molecule.
For ionic compounds, formula tell
the simples ratio of actions and
anions.
Molecular Weight ?
Molecular compounds
Formula Weight?
Ionic compounds
CHEM 100, Fall 2013 LA TECH
3-9
CHEM 100, Fall 2013 LA TECH
3-10
CHEM 100, Fall 2013 LA TECH
3-11
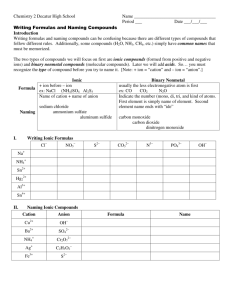
Nomenclatuere: Naming Compounds
•
Ionic compounds
• Molecular compounds(Inorganic & organic)
• Acids and bases
• Hydrated compounds
CHEM 100, Fall 2013 LA TECH
3-12
Formation of Ionic Compound, NaCl
CHEM 100, Fall 2013 LA TECH
3-13
Ionic Compounds
Characteristics of compounds with ionic bonding:
Compound of metal and non-metal
Composed of ions: cation and anion
non-volatile, thus high melting points
solids do not conduct electricity, but melts (liquid
state) do
many, but not all, are water soluble
CHEM 100, Fall 2013 LA TECH
3-14
Electrical
Conductivity of
Ionic Solution
Electrolytes
Aqueous solutions conducts
electricity
strong-electrolytes
weak-electrolytes
Non-electrolytes
Aqueous solutions do not
conducts electricity
CHEM 100, Fall 2013 LA TECH
3-15
Ionic Compounds
Type I ionic compound
(fixed charge) NaCl
CHEM 100, Fall 2013 LA TECH
Type II ionic compound
FeCl2 and FeCl3,
SnCl2 and SnCl4
3-16
Type I
CHEM 100, Fall 2013 LA TECH
Type II
3-17
Charges on Some Common
Monatomic Cations and Anions
CHEM 100, Fall 2013 LA TECH
3-18
Charge on Metal Ions
Monatomic Ions (Type I)
Group IA
+1 Group A #
Group IIA
+2 Group A #
Non-metals
Group IIB
-1 (8 - Group B #)
Monatomic Ions (Type II)
Transition metal ionic compounds:
have ions with different charges
E.g. Iron :Fe2+ and Fe3+
CHEM 100, Fall 2013 LA TECH
3-19
Some Common Polyatomic Ions
with Their Charge
Name
Formula Name
Formula
acetate
carbonate
hydrogen carbonate
(aka bicarbonate)
hydroxide
nitrate
C2H3O2–
CO32–
hypochlorite
chlorite
ClO–
ClO2–
chlorate
perchlorate
ClO3–
ClO4–
nitrite
chromate
NO2–
CrO42–
sulfate
SO42–
sulfite
SO32–
hydrogen sulfate
HSO4–
(aka bisulfate)
dichromate
ammonium
Cr2O72–
NH4+
hydrogen sulfite
(aka bisulfite)
CHEM 100, Fall 2013 LA TECH
HCO3
–
OH–
NO3–
HSO3–
3-20
1) Identify the types of ions in the following list:
F-,Fe2+, Fe3+,Ca2+,H3O+,Ba2+,Cl-,Cu+, Cu2+, Sr2+,Ra2+,Ni2+, Ni4+,
Br-,CrO42-, Cr2O72-, MnO4-, C2O42-, NH4+,
a) Cations: Type I :
b) Cations: Type II :
c) Monoatomic anions:
d) Polyatomic anions:
e) Polyatomic cations:
CHEM 100, Fall 2013 LA TECH
3-21
Polyatomic Ions
More than one atom joined together
have negative charge except for NH4+
and its relatives
negative charges range from
-1 to -4
Table in the Book
CHEM 100, Fall 2013 LA TECH
3-22
2) Give the names of the following ions:
a) Cl-
b) SO42-
c) SO32-
d) Fe3+
e) Sr2+
f) CO3-2
g) NO3-
h) PO43-
i) Hg2+
j) Hg22+
k) Cr2O72-
l) MnO4-
m) C2O42-
n) NH4+,
o) U4+
p) HCO3-
q) C2H3O2- or
CH3COO-
r) HSO3-
s) HPO4-
t) H2PO4-
CHEM 100, Fall 2013 LA TECH
3-23
3) Give the name of ion and the name and
formula of acid it came from
Ion
Ion name
Acid
formula
Acid
name
Ion
a) ClO-
i) IO-
b) ClO2-
j) IO2-
c) ClO3-
k) IO3-
e) ClO4-
l) IO4-
f) SO4-2
m) SeO4-2
g) C2H3O2-
n)BrO3-
CHEM 100, Fall 2013 LA TECH
Ion name
3-24
Naming Ionic Compounds:
Metal + Nonmetal: Main Group (“p” Block) Metals
•
Alkali and Alkaline Earth Metals
– Metal name first, followed by
nonmetal
– Nonmetal ending is exchanged
with “IDE”
Examples:
– MgCl2: magnesium chloride
– KNO3: potassium nitrate
• Alkali (+1) and alkaline earth (+2)
metals’ oxidation states are
known.
– That is why their charge is NOT
indicated in the formula name!
CHEM 100, Fall 2013 LA TECH
3-25
Naming Ionic Compounds:
Metal + Nonmetal for Transition and Main Group
(“p” Block) Metals
• Metal name first, followed by nonmetal
• Metal’s oxidation state is indicated by a Roman numeral.
• Nonmetal ending is exchanged with “IDE.”
• Examples:
» MnBr4:
» Fe2O3:
» SnF2:
CHEM 100, Fall 2013 LA TECH
manganese (IV) bromide
iron (III) oxide
tin (II) fluoride
3-26
Names of Ionic Compounds
1. Name the metal first.
If the metal has more than one oxidation state, the
oxidation state is specified by Roman numerals in
parentheses.
2. Then name the non-metal,
changing the ending of the non-metal to -ide.
CHEM 100, Fall 2013 LA TECH
3-27
Ionic compounds
Some simple ions
Cations
Anions
Na
Cl
-
+
Mg
O
2+
2-
Al
N
3+
3-
Exchange charge as subscripts on the metal and nonmetal
Formula for some ionic compounds
NaCl
MgCl2
Na2O
MgO
Na3N
Mg3N2
CHEM 100, Fall 2013 LA TECH
AlCl3
Al2O3
AlN
Give the simple ratio
3-28
Nomenclature
NaCl
Fe2O3
NH4NO3
KClO4
CaCO3
NaOH
AgNO3
Mg(C2H3O2)2
Co2(SO4)3
KI
Mg3N2
CHEM 100, Fall 2013 LA TECH
NaCl sodium chloride
Fe2O3 iron(III) oxide
NH4NO3 ammonium nitrate
KClO4 potassium perchlorate
CaCO3 calcium carbonate
NaOH sodium hydroxide
AgNO3 silver nitrate
Mg(C2H3O2)2 magnesium acetate
Co2(SO4)3 cobalt(III) sulfate
KI potassium iodide
Mg3N2 magnesium nitride
3-29
4) Give the formula of following ionic compounds
silver
carbonate
potassium
hydrogen
phosphate
aluminum
hydroxide
sodium bicarbonate
calcium
acetate
potassium
permanaganate
calcium
perchlorate
magnesium
hydrogen sulfite
sodium
hypochlorite
iron(II) carbonate
Iron(II)chloride
nonahydrate
barium oxide
CHEM 100, Fall 2013 LA TECH
3-30
5) Write the correct formula for:
tin(IV) chlorite
mercury(II)
phosphate
copper(I) sulfite stannous
dichromate
potassium
perchlorate
barium iodate
tin(II)
hydrogen
sulfide
CHEM 100, Fall 2013 LA TECH
tin(II)
carbonate
mercurous
acetate
lead(II) chromate
iron(III) nitrate
ferric sulfate
ferrous
hydroxide
lead(IV)
hydrogen
phosphate
aluminum
sulfate
iron(II)
bicarbonate
magnesium
dihydrogen
phosphate
plumbous
cyanide
silver phosphate
3-31
Hydrated Compounds vs. Anhydrous Compounds
Hydrate
CoCl2∙6H2O
CHEM 100, Fall 2013 LA TECH
Anhydrous
CoCl2
3-32
Hydrated Compounds
•
Prefix
No. of
Waters
hemi
½
mono
1
di
2
tri
3
tetra
4
penta
5
hexa
6
hepta
7
octa
8
Hydrates are ionic compounds containing a
specific number of waters for each formula unit.
•
•
•
Water of hydration is often “driven off” by
heating.
In formula, attached waters follow
– CoCl2•6H2O
In name, attached waters are indicated by
prefix + hydrate after name of ionic compound.
– CoCl2•6H2O = cobalt(II) chloride hexahydrate
– CaSO4•½H2O = calcium sulfate hemihydrate
CHEM 100, Fall 2013 LA TECH
3-33
Problem:
1. What is the formula of magnesium sulfate heptahydrate?
2. What is the name of NiCl2•6H2O?
CHEM 100, Fall 2013 LA TECH
3-34
Answers:
1. What is the formula of magnesium sulfate
heptahydrate?
2+
2−
Mg + SO4
MgSO4
MgSO47H2O
2. What is the name of NiCl2•6H2O?
2+
−
Ni + 2 Cl
nickel(II) chloride
nickel(II) chloride hexahydrate
CHEM 100, Fall 2013 LA TECH
3-35
CHEM 100, Fall 2013 LA TECH
3-36
6) Give the chemical name or the formulas for the
following ionic compounds:
UO2
Co3(PO4)2
cobaltic nitrate
magnesium
dihydrogen
phosphate
CaCl22H2O
ammonium ferrous
sulfate hexahydrate
CHEM 100, Fall 2013 LA TECH
3-37