LMNA and its role in Hutchinson-Gilford Progeria Syndrome (HGPS)
advertisement

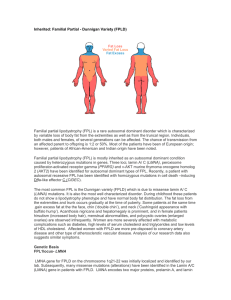
LMNA and its role in HutchinsonGilford Progeria Syndrome (HGPS) Peter St. Andre Outline • Background – Overview of HGPS – Discovery and role of LMNA in HGPS • Gene and protein data – Homology, phylogeny,domains, ontology, and interactions • Further directions – New angles at research – Current drug trials Background on disease • Characterized by premature accelerated aging beginning between 6-12 months of age • There are around 100 living patients worldwide at a given time • Often fatal by the age of 13 with no known cure maldova.org Quick facts about HGPS • Symptoms include: hair loss, scleroderma, severe arthritis, etc. • Differs from other known progeroid diseases • Fastest time from discovery to drug trials Susana Lopez, 21, is the oldest known sufferer of HGPS health.tbo.com Cause of HGPS • Able to discover cause of disease incredibly quickly • de novo mutations occur in exon 11 of LMNA gene • >70% are 1824C>T (G608G) mutations, with ~10% 1821G>A (V607V) • LMNA produces lamin A/C proteins and through alternate splicing three isoforms can be created • Mutation does not allow for cleavage to produce a mature functional lamin A Mutation behind HGPS Protein motifs and domains • 9 aa seq. recognized as IF domain -IHAYRKLLEIF protein • Sequences returned expected motifs and accompanying domains • Main components of nuclear lamins • Tail domain is contains NLS Images taken from: Pfam IF tail domain Homology •Lmn-1 is homolog in C. elegans •Only lamin in C. elegans, predecessor of all lamins? Homo sapiens 664 aa C. elegans 566 aa Phylogeny •Differences between branches is very small •Zebrafish still has 63% identity •Run through several programs all returned exact phylogeny Gene ontology Molecular Function Biological Process Cellular Component • Protein binding • Muscle development • Lamin filament • Structural molecular activity • More associated with Charcot-Emery disease • Nuclear envelope • Perinuclear region of cytoplasm Protein interactions Mouse Human • ZMPSTE24 could be a focus • Mutations of this protein cause similar phenotypes when compared to HGPS FTI clinical trials • Drug trial began in 2007 and are expected to finish later this year • While there is evidence in mice of rescuing phenotypes, in humans FTIs seem unlikely to solve anything • Patients symptoms become too severe too quickly • Still have a dysfunctional lamin A protein Further research • FTIs offer promise and a start • Run 2D-gel analyses as lamins are characterized by pI • Focus on the post-translational modifications • Key would be to induce cleavage of farnesylated end therefore producing mature lamin A Special thanks to: Dr. Ahna Skop Dr. Robert Goldman Peers References Eriksson, M., Brown, W. T., Gordon, L. B., Glynn, M. W., Singer, J., Scott, L., Erdos, M. R., Robbins, C. M., Moses, T. Y., Berglund, P., Dutra, A., Pak, E., Durkin, S., Csoka, A. B., Boehnke, M., Glover, T. W., & Collins, F. S. (2003). Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature, 423, 293-298. doi: 10.1038/nature01629 Meta, M., Yang, S. H., Bergo, M. O., Fong, L. G., & Young, S. G. (2006). Protein farnesyltransferase inhibitors and progeria. Trends in Molecular Medicine, 12(10), 480-487. doi: 10.1016/j.molmed.2006.08.006 Sagelius, H., Rosengardten, Y., Hanif, M., Erdos, M., Rozell, B., Collins, F., & Eriksson, M. (2008). Targeted transgenic expression of the mutation causing Hutchinson-Gilford progeria syndrome leads to proliferative and degenerative epidermal disease. Journal of Cell Science, 121, 969-978. doi: 10.1242/jcs.022913 Ruchaud, S., Korfall, N., Villa, P., Kottke, T., Dingwall, C., Kaufmann, F., Earnshaw,, W. (2002). Caspase-6 gene disruption reveals a requirement for lamin A in apoptotic chromatin decondensation. The EMPBO Journal (21). Retrieved from: The EMBO Journal. Moulson CL, Fong LG, Gardner JM, Farber EA, Go G, Passariello A, Grange DK, Young SG, Miner GH. (2007). Increased progerin expression associated with unusual LMNA mutations causes severe progeroid syndromes. Human Mutations 28(9). PMID: 17469202.