Notes packet - Ms. Hanna's Science Class
advertisement

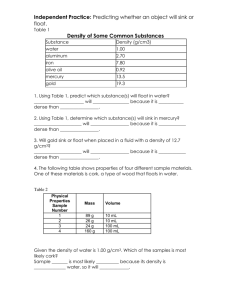
Name _______________________________________ Physical Science Date _______________ Ms. Hanna Substances have characteristic properties 1. Properties can be used to _______________________ substances. 2. Properties may be used to separate a mixture into its components (parts). A. ____________________ are physical combinations of materials and can be separated by physical means Ex: How can you separate spices from iron filings? Ex: How can you separate salt water into salt and water? 3. Physical Properties A. _________________ – the ability of a substance to dissolve in another substance Solute-Substance being dissolved (sugar, salt, kool-aid) Solvent-Substance doing the dissolving (water) What can affect solubility? B. __________________– A force of attraction and repulsion when electrical fields line up The ends of a magnet are called its ______________ The ____________ magnetic force occurs at the poles Poles are labeled ____________ and _____________ Like poles(N-N or S-S) ____________, unlike poles (N-S) ____________ Magnetic Field A region around a magnet where the force is the strongest The __________________ of the field decreases as the distance from the field increases. C. Density is a ______________________ between an objects ______________ and it's _____________________. Mass = The amount of ________________________in an object. Weight: A measure of the pull of ___________________________on an object. Volume: The amount of ______________________an object takes up. Density = Ex. The mass of an object is 25grams. The volume of an object is 5 cm3. What is its density? F= S= A= Molecular structure of ___________________ ________________________ The more tightly packed the molecules are the ________________ dense the object is Therefore ________________________ is the most dense Example: Density Cube lab What is the density of an irregular object? ____________________________ You must use a ________________________________ The units are not cm3 they are _______________ Ex. What is the density of a hammer that has a mass of 235 g? (This graduated cylinder measures in L not ml) Volume of hammer F: volume = Total V-liquid V Density of hammer F: density = mass/volume S= A= If you had two rods the same size (volume), but one was made up calcium and the other one made of sulfur which one would have the greater density? Their molecule size is seen below. How can you tell if an object will float or sink? You need to know the density of ____________________________ What water has the greater density? The beaker full of water or the beaker filled with one eye dropper of water? Find the density of water 1) Look at your values for density in your chart. Does the density of the different volumes of water seem to be about the same? 2) What do you think is the density of water in g/cm3? 3) Using the data from your chart, graph the volume and mass for 100 mL, 50 mL, and 25 mL. 4) Look at the graph you made. If you measured 40 milliliters of water, what do you think its mass would be? What would its density be? Volume: 40 mL Mass ___________ Density _________ 5) Choose any volume of water between 1 and 100 milliliters. Based on the graph, what would its mass be? What would its density be? Volume _________ Mass ___________ Density _________ The _________________________ of a liquid determines whether it will float on or sink in another liquid. • A liquid will float if it is ________________________________than the liquid it is placed in. • A liquid will sink if it is _____________________________than the liquid it is placed in. Water has a density of ______________________ Will it float, neither float nor sink, or sink? Ex. Do raisins have a greater density than soda? Materials: raisins (about 12) 2 glass clear beaker Clear soda (like 7up, Sierra Mist or Sprite) Hot plate Hypothesis: _______________________________________________________________________________________ _________________________________________________________________________________________________ __________________________________________________________________________________________________ Procedure: 1) Pour clear soda into a medium size beaker and add some raisins (fresh). Observations: What happens?? 2) At first what had the larger density, the soda or the raisins? _______________________ 3) After a minute, which had the larger density? ______________________________ 4) Why do the raisins appear to be dancing? 5) What happens when raisins are put into hot soda? Explain why What is the most dense? Label the picture to the right. Can you change the density of water? Why do boats float? buoyancy: arises from the fact that _________________________________increases with depth and from the fact that the increased pressure is exerted in all directions (__________________________________) so that there is an unbalanced upward force on the bottom of a submerged object Buoyant force opposes the force of _____________________ Do the piece of aluminum sink float activity What has a greater density, salt water or fresh? Draw a carrot slice in each of the cups below to reflect your observations 1)Will a boat float higher or lower in the ocean? 2) How does adding salt change the density of the water? 3) What would you expect if you placed equal volumes of water and saltwater on opposite ends of a balance? 4) Adding salt to water increases both its mass and volume; which do you think it increases more, the mass of the water or the volume? REAL LIFE EXAMPLES OF BUOYANCY IN ACTION Ex. __________________________________ swim bladder has an ________________________ in it Relax their muscles --> sac increases in size --> displaces more water --> Contracts their muscles --> sac decreases in size --> displaces less water --> Ex. ______________________________________________ Decreasing water in the ballast tanks--> Increasing water in the ballast tanks --> Show The Cartesian Diver demo Can temperature change the density of a liquid?? Hypothesis: What is more dense? Cold or warm water? __________________________________________________________________________________________________ __________________________________________________________________________________________________ Watch the video to answer the questions www.middleschoolchemistry.com/multimedia/chapter3/lesson6#hot_water_on_cold water 1. Draw what you observed in the cup of room-temperature water after adding blue cold water and yellow hot water. 2. Be sure to label the areas of cold and hot water. Is cold water more, less, or the same density as room-temperature water? Is hot water more, less, or the same density as room-temperature water Look at the model of water molecules in the diagram below to help you compare the volume, mass, and density of cold and hot water. Write more, less, or same in the chart to describe the volume, mass, and density of cold and hot water compared to room temperature water. Use what you know about density to answer the following questions. Why does cold water sink in room-temperature water? Why does hot water float on room-temperature water? Heating a substance causes molecules to _________________________________and spread slightly further apart, occupying a larger volume that results in a ________________________in density. (Mass stays the same) • Cooling a substance causes molecules to _____________________________and get slightly closer together, occupying a smaller volume that results in an ________________________ in density. • Hot water is less dense and will ________________________on room-temperature water. • Cold water is more dense and will ____________________ in room-temperature water.