Chapter 3 - Key Concepts
advertisement

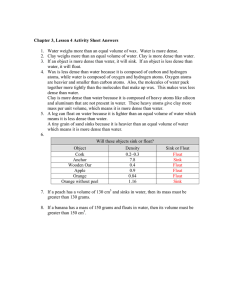
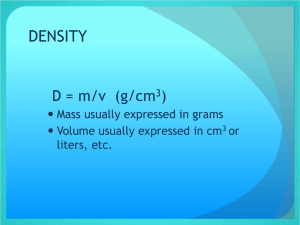
Chemistry – Properties and Interactions of Matter Chapter 3: Key Concepts Lesson 3.1: What is Density? Density is a characteristic property of a substance. The density of a substance is the relationship between the mass of the substance and how much space it takes up (volume). The mass of atoms, their size, and how they are arranged determine the density of a substance. Density equals the mass of the substance divided by its volume; D = m/v. Objects with the same volume but different mass have different densities. Lesson 3.2: Finding Volume: The Water Displacement Method A submerged object displaces a volume of liquid equal to the volume of the object. One milliliter (1 mL) of water has a volume of 1 cubic centimeter (1cm3). Different atoms have different sizes and masses. Atoms on the periodic table are arranged in order based on the number of protons in the nucleus. Even though an atom may be smaller than another atom, it might have more mass. The mass of atoms, their size, and how they are arranged determine the density of a substance. Density equals the mass of the object divided by its volume; D = m/v. Objects with the same mass but different volume have different densities. Lesson 3.3: Density of Water Just like solids, liquids also have their own characteristic density. The volume of a liquid can be measured directly with a graduated cylinder. The molecules of different liquids have different size and mass. The mass and size of the molecules in a liquid and how closely they are packed together determine the density of the liquid. As in a solid, the density of a liquid equals the mass of the liquid divided by its volume; D = m/v. The density of water is 1 gram per cubic centimeter. The density of a substance is the same regardless of the size of the sample. Lesson 3.4: Density: Sink and Float for Solids The density of an object determines whether it will float or sink in another substance. An object will float if it is less dense than the liquid it is placed in. An object will sink if it is more dense than the liquid it is placed in. Lesson 3.5: Density: Sink and Float for Liquids Since density is a characteristic property of a substance, each liquid has its own characteristic density. The density of a liquid determines whether it will float on or sink in another liquid. A liquid will float if it is less dense than the liquid it is placed in. A liquid will sink if it is more dense than the liquid it is placed in. Lesson 3.6: Temperature Affects Density Heating a substance causes molecules to speed up and spread slightly further apart, occupying a larger volume that results in a decrease in density. Cooling a substance causes molecules to slow down and get slightly closer together, occupying a smaller volume that results in an increase in density. Hot water is less dense and will float on room-temperature water. Cold water is more dense and will sink in room-temperature water.