21.7 The High Specific Heat Capacity of Water
advertisement

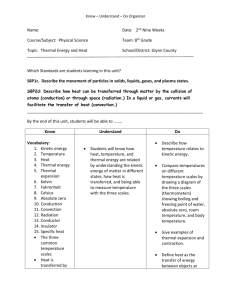
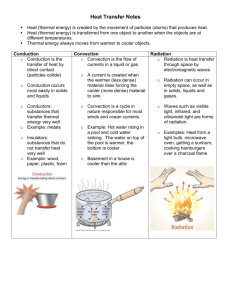
Thermal Energy Internal energy: energy of the moving particles that compose matter Starter 3 Thermal Energy Transfer • Read Ch. 22.1-22.3 • Fold a piece of notebook paper to form three columns – Head each column with one of the three ways that thermal energy can be transferred – Define each – List three main points from the reading for each thermal energy transfer process • On the back of the paper, prepare an example illustrating how each transfers heat Today’s Key Terms and Ideas • • • • • Thermal Energy Kinetic Theory Heat Thermal Equilibrium and heat transfer Kinetic Theory as it relates to expansion and contraction • Hot vs. cold Physics and Particles • Particle is a general term used to describe molecules, atoms and sub-atomic particles 21.1 Temperature The higher the temperature of a substance, the faster the motion of its molecules. This is also referred to as the Kinetic Theory— a) all matter is made of atoms and molecules that are moving. b) The higher the temperature, the faster the particles move. c) Given the same temperature, heavier particles move slower than lighter particles. Matter is changing state solid melting freezing liquid evaporation condensation gas Particle speed is increasing Increasing Avg. KE Increasing Temp. 21.1 Temperature Temperature and Kinetic Energy Temperature is related to the average kinetic energy of the atoms and molecules in a substance. The faster the molecules move, the greater ______________ the temperature and the greater _____________ the average kinetic energy and the greater __________ the particle speed. 21.2 Heat 1. Heat is the quantity of thermal energy transferred 2. Heat always flows from a substance with a higher temperature to a substance with a lower temperature. 3. Heat flows only when there is a difference in temperature. 4. Heat units are calories or joules. 21.2 Heat Just as water will not flow uphill by itself, regardless of the relative amounts of water in the reservoirs, heat will not flow from a cooler substance into a hotter substance by itself. hotter Entropy! Flow from higher to lower energy state. colder 21.2 Heat What causes heat to flow? A difference in temperature between objects in thermal contact. 21.4 Internal Energy When a substance takes in or gives off heat, its internal energy changes. 21.3 Thermal Equilibrium What happens when a warmer substance comes in contact with a cooler substance? • Heat flows between two objects of different temperature until they have the same temperature. • The loss of thermal energy from the warmer object equals the gain of thermal energy for the cooler object 21.8 Thermal Expansion Most forms of matter—solids, liquids, and gases— expand when they are heated and contract when they are cooled. 21.8 Thermal Expansion When the temperature of a substance is increased, its molecules jiggle faster and normally tend to move farther apart. This results in an expansion of the substance. • Gases generally expand or contract much more than liquids. • Liquids generally expand or contract more than solids. Starter Question #2 How does a thermometer work? The kinetic theory be used to explain expansion and contraction of materials when the temperature of the material changes. As the temperature rises, heat is transferred from the surroundings to the liquid inside the thermometer and the molecules that compose the liquid vibrate faster. This causes the liquid to expand and rise. As the temperature falls, heat is transferred away from the liquid inside to the surroundings and the molecules that compose this liquid slow down. This causes the liquid to contract. • The liquid in the thermometer stops rising or falling when thermal equilibrium is reached (no more heat flow!) Air temperature = Liquid temperature 21.6 Specific Heat Capacity Do copper, clay and water have the same chemical composition? •No. Copper is composed of Cu atoms and water is composed of H2O molecules. Clay is a complex silicate. •The difference in chemical composition influences how copper, clay and water respond when heat is transferred. The specific heat capacity of a substance is the quantity of heat required to raise 1 g of a substance by 1 degree Celsius. 21.6 Specific Heat Capacity A substance with a high specific heat capacity can absorb a large quantity of heat before it will raise in temperature (water has a high specific heat). A substance with a low specific heat requires relatively little heat to raise its temperature (copper has a low specific heat). 21.6 Specific Heat Capacity highest lowest 21.6 Specific Heat Capacity think! Which has a higher specific heat capacity—water or sand? Explain. 21.6 Specific Heat Capacity think! Which has a higher specific heat capacity—water or sand? Explain. Answer: Water has a greater heat capacity than sand. Water is much slower to warm in the hot sun and slower to cool at night. Sand’s low heat capacity, shown by how quickly it warms in the morning and how quickly it cools at night, affects local climates. Good conductors have a low specific heat capacity! 21.6 Specific Heat Capacity A gram of water requires 1 calorie of energy to raise the temperature 1°C. It takes only about one eighth as much energy to raise the temperature of a gram of iron by the same amount. The capacity of a substance to store heat depends on its chemical composition. 21.6 Specific Heat Capacity 6. What is the difference between a substance with a high specific heat and a low specific heat capacity? • Substances with a low specific heat (e.g., metals) need very little heat to raise temperature – Good conductors, not good absorbers, do not hold onto heat well • Substances with a high specific heat need a large quantity of heat to raise temperature. – Poor conductors, good absorbers, store and hold onto heat well 7. How does the specific heat of water help to moderate climate? • During the summer, surrounding air is cooled by the water and keeps the coast cooler than the intercontinental locations. • During the winter, the surrounding air is warmed by the water and keeps the coast warmer than the intercontinental locations. 21.7 The High Specific Heat Capacity of Water The property of water to resist changes in temperature improves the climate in many places. 21.7 The High Specific Heat Capacity of Water Water has a high specific heat and is transparent, so it takes more energy to heat up than land does. 21.7 The High Specific Heat Capacity of Water Water’s capacity to store heat affects the global climate. Water stores and hold heat well because of its high specific heat. •Gulf Stream brings warm water northeast from the Caribbean. •It holds much of its thermal energy long enough to reach the North Atlantic off the coast of Europe. •As it cools, the energy released is carried by the prevailing westerly winds over the European continent. The Gulf Stream brings warm winters to Ireland and the prevailing winds off the Atlantic carry with them rain. It means grass can grow almost all year round — creating the lush sweeping pastures of the Emerald Isle. Today they make up 93 percent of all farmland. No other country in Europe has quite as much grass as Ireland. 21.7 The High Specific Heat Capacity of Water Climate of Europe Look at a world globe and notice the high latitude of Europe. Both Europe and Canada get about the same amount of the sun’s energy per square kilometer. Marine Climate Continental Climate Cork Edmonton Same insolation angle, different climate due to proximity to water and the warming effect from the Gulf Stream http://www.sampleireland.com/weather-in-ireland-year-round.html 21.7 The High Specific Heat Capacity of Water Climate of America On the west coast, air moves from the Pacific Ocean to the land. • In winter, the water warms the air that moves over it and warms the western coastal regions of North America. • In summer, the water cools the air and the western coastal regions are cooled. The central interior of a large continent usually experiences extremes of temperature. Land, with a lower specific heat capacity, gets hot in summer but cools rapidly in winter. 8. In which three ways can the thermal energy (or heat) of a substance be transferred? Heat can be transferred by conduction, by convection, and by radiation. 22.1 Conduction In conduction, collisions between particles transfer thermal energy, without any overall transfer of matter. 22.1 Conduction Heat from the flame causes atoms and free electrons in the end of the metal to move faster and jostle against others. The energy of vibrating atoms increases along the length of the rod. 22.2 Convection In convection, heat is transferred by movement of the hotter substance from one place to another. 22.2 Convection Convection occurs in all fluids. a. Convection currents transfer heat in air. Hot, less dense fluid rises in the presence of cooler, more dense fluid. 22.2 Convection Convection occurs in all fluids. a. Convection currents transfer heat in air. b. Convection currents transfer heat in liquid. When fluid particles at the bottom of the pan begin to vibrate faster, they expand and decrease in density, making the hotter fluid more buoyant. 22.3 Radiation In radiation, heat is transmitted in the form of radiant energy, or electromagnetic waves. 22.3 Radiation Most of the heat from a fireplace goes up the chimney by convection. The heat that warms us comes to us by radiation. • Radiation is caused by moving electrons or charged particles in matter. The faster the particles move, the higher the frequency of the electromagnetic radiation. 22.3 Radiation a. Radio waves send signals through the air. 22.3 Radiation a. Radio waves send signals through the air. b. You feel infrared waves as heat. 22.3 Radiation a. Radio waves send signals through the air. b. You feel infrared waves as heat. c. A visible form of radiant energy is light waves. 11. What happens to the frequency of radiant energy as the temperature of the substance increases or decreases? • The frequency of radiant energy increases as the temperature of the substance increases. http://mail.jsd.k12.ca.us/bf/bflibrary/images/electromagnetic-spectrum.jpg