summary
advertisement

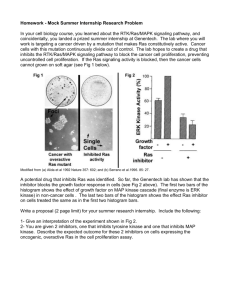
Chapter 16, see p330 in text book Cell-to-Cell Signaling: Hormones and Receptors signaling molecules receptors signal transduction Overview of Extracellular Signaling (1) synthesis and (2) release of the signaling molecule; (3) transport of the signal to the target cell; (4) detection of the signal by a specific receptor protein; (5) a change in cellular metabolism, function, or development triggered by the receptor-signal complex; (6) removal of the signal, which often terminates the cellular response. 1.1 Signal molecules: Signaling Molecules Operate over Various Distances in Animals 1) endocrine, 2) paracrine, or autocrine 3) Synaptic signal In endocrine signaling, signaling molecules, called hormones, act on target cells distant from their site of synthesis by cells of endocrine organs. Receptor Proteins Exhibit Ligand-Binding and Effector Specificity Hormones Can Be Classified Based on Their Solubility and Receptor Location Most hormones: (1) small lipophilic molecules that diffuse across the plasma membrane and interact with intracellular receptors; and (2) hydrophilic or (3) lipophilic molecules Lipophilic Hormones with Intracellular Receptors steroids (cortisol, progesterone, estradiol, and testosterone), thyroxine, and retinoic acid Water-Soluble Hormones with Cell-Surface Receptors (1)peptide hormones, such as insulin, growth factors, and glucagon, and (2) small charged molecules, such as epinephrine and histamine Lipophilic Receptors Hormones with Cell-Surface Cell-Surface Receptors Belong to Four Major Classes G protein coupled receptors Ion-channel receptors Tyrosine kinase linked receptors Receptors with intrinsic enzymatic activity Effects of Many Hormones Are Mediated by Second Messengers second messengers,intracellular signaling molecules, cAMP; cGMP; 1,2-diacylglycerol (DAG); IP3; various inositol phospholipids (phosphoinositides); and Ca2+. Other Conserved Proteins Function in Signal Transduction GTPase Switch Proteins: there are two classes of GTPase switch proteins, and monomeric Ras and Ras-like proteins. Protein Kinases Adapter Proteins Common Signaling Pathways Are Initiated by Different Receptors in a Class The Synthesis, Release, and Degradation of Hormones Are Regulated Peptide Hormones and Catecholamines Steroid Hormones, Thyroxine, and Retinoic Acid Feedback Control of Hormone Levels SUMMARY signaling molecules, membrane-anchored and secreted proteins, lipophilic and hydrophilic molecules, and gases. Binding of extracellular signaling molecules to cell-surface receptors trigger intracellular pathways that modulate cellular metabolism, function, or development. The second messengers, such as Ca2+, cAMP, and IP3 ; Conserved proteins in signal-transduction pathways include GTPase switch proteins, protein kinases, and adapter proteins. Extracellular signals are often integrated into complex regulatory networks in which the synthesis, release, and degradation of hormones are precisely regulated. Identification and Purification of CellSurface Receptors Binding of a hormone to a receptor involves of weak interactions ionic and van der Waals bonds and hydrophobic interactions. Hormone Receptors Are Detected by Binding Assays The KD, the hormone concentration at which the receptor is half-saturated, also can be calculated from the specific binding curve. KD Values for Cell-Surface Hormone Receptors Approximate the Concentrations of Circulating Hormones Affinity Techniques Permit Purification of Receptor Proteins Many Receptors Can Be Cloned without Prior Purification SUMMARY Receptors bind to ligands. This specificity is determined by interactions between ligand determinants and specific amino acids in the receptor. Receptors can be purified directly using ligands as affinity reagents. In some cases, the genes encoding receptors for specific ligands can be isolated from cDNA libraries transfected into cultured cells. Cells expressing the receptor are detected using labeled ligand as a probe. G Protein Coupled Receptors and Their Effectors cell-surface receptors --- trimeric signal--transducing G protein---effector enzyme --- an intracellular second messenger All G protein coupled receptors (GPCRs) contain seven membrane-spanning regions with N-terminal segment on the exoplasmic face and C-terminal segment on the cytosolic face of the plasma membrane. Hormone—receptor—G protein—enzyme—the second message—kinase—enzyme or functional protein—biological effect Binding of Epinephrine to Adrenergic Receptors Induces Tissue-Specific Responses Stimulation of b-Adrenergic Receptors Leads to a Rise in cAMP Critical Features of Catecholamines and Their Receptors Have Been Identified Trimeric Gs Protein Links b-Adrenergic Receptors and Adenylyl Cyclase b-adrenergic receptors, which are coupled to Gs, or stimulatory G protein b-adrenergic receptors is an elevation in the intracellular level of cAMP. Cycling of Gs between Active and Inactive Forms The G proteins and other GPCRs contain three subunits designated a, b, and g. GTPase switch proteins alternate between an "on" state with bound GTP and an "off" state. Binding of a hormone or agonist to the receptor changes its conformation, causing it to bind to the trimeric Gs protein in such a way that GDP is displaced from Gsa and GTP is bound. The Gsa ·GTP complex, which dissociates from the Gbg complex, then binds to and activates adenylyl cyclase. Amplification of Hormone Signal Termination of Cellular Response Some Bacterial Toxins Irreversibly Modify G Proteins Adenylyl Cyclase Is Stimulated and Inhibited by Different Receptor-Ligand Complexes GTP-Induced Changes in Gsa Favor Its Dissociation from G b g and Association with Adenylyl Cyclase Gia and Gsa Interact with Different Regions of Adenylyl Cyclase Degradation of cAMP Also Is Regulated SUMMARY Many cell-surface receptors, seven transmembrane domains - trimeric G proteins. All G proteins contain three subunits: a, b, and g. The intrinsic GTPase activity of G a inactivates G a · GTP by catalyzing GTP hydrolysis: Pi is released and the resulting G a · GDP then dissociates from its effector and reassociates with G b g . Adenylyl cyclase, which catalyzes the formation of cAMP from ATP, is the best-characterized effector regulated by trimeric G proteins. All adenylyl cyclase isoforms are stimulated by Gsa , but only specific isoforms are inhibited by Gia and G b g . Gsa , Gia , and G b g interact with different regions of the catalytic domain of adenylyl cyclase. Receptor Tyrosine Kinases and Ras receptor tyrosine kinases (RTKs) , The ligands for RTKs are soluble or membrane-bound peptide/protein hormones including nerve growth factor (NGF), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), epidermal growth factor (EGF), and insulin. All RTKs comprise an extracellular domain containing a ligand-binding site, a single hydrophobic transmembrane a helix, and a cytosolic domain that includes a region with protein-tyrosine kinase activity. Binding of ligand causes most RTKs to dimerize; the protein kinase of each receptor monomer then phosphorylates a distinct set of tyrosine residues in the cytosolic domain of its dimer partner, a process termed autophosphorylation. The phosphotyrosine residues in activated RTKs interact with adapter proteins containing SH2 or PTB domains. Ras and G a Subunits Belong to the GTPase Superfamily of Intracellular Switch Proteins Activation of both Ras and G a is triggered by hormone binding to an appropriate cell-surface receptor. Ras activation is accelerated by a protein called guanine nucleotide exchange factor (GEF), which binds to the Ras · GDP complex, causing dissociation of the bound GDP. Hydrolysis of the bound GTP deactivates both Ras and G a . The average lifetime of a GTP bound to Ras is about 1 minute, which is much longer than the lifetime of G a · GTP. The reason for this difference is that the deactivation of Ras, unlike the deactivation of G a , requires the assistance of another protein: a GTPase-activating protein (GAP), which binds to Ras · GTP and accelerates its intrinsic GTPase activity by a hundredfold. An Adapter Protein and GEF Link Most Activated RTKs to Ras SH2 Domain in GRB2 Adapter Protein Binds to a Specific Phosphotyrosine in an Activated RTK Sos, a Guanine Nucleotide Exchange Factor, Binds to the SH3 Domains in GRB2 SUMMARY Receptor tyrosine kinases (RTKs), bind to peptide hormones, may exist as dimers or dimerize during binding to ligands. Ligand binding leads to activation of the kinase activity of the receptor and autophosphorylation of tyrosine residues. Ras is an intracellular GTPase switch protein that acts downstream from most RTKs. Like Gsa , Ras cycles between an inactive GDPbound form and active GTP-bound form. Unlike GPCRs, which interact directly with an associated G protein, RTKs are linked indirectly to Ras via two proteins, GRB2 and Sos. The SH2 domain in GRB2, an adapter protein, binds to specific phosphotyrosines in activated RTKs. Normally, Ras activation and the subsequent cellular response is induced by ligand binding to an RTK. However, in cells that contain a constitutively active Ras, the cellular response occurs in the absence of ligand binding. MAP Kinase Pathways Activated Ras then induces a kinase cascade that culminates in activation of MAP kinase. This serine/threonine kinase, which can translocate into the nucleus, phosphorylates many different proteins including transcription factors that regulate expression of important cell-cycle and differentiation-specific proteins. Signals Pass from Activated Ras to a Cascade of Protein Kinases Ksr May Function as a Scaffold for the MAP Kinase Cascade Linked to Ras Phosphorylation of a Tyrosine and a Threonine Activates MAP Kinase Various Types of Receptors Transmit Signals to MAP Kinase Multiple MAP Kinase Pathways Are Found in Eukaryotic Cells Specificity of MAP Kinase Pathways Depends on Several Mechanisms SUMMARY Activated Ras promotes formation of signaling complexes at the membrane containing three sequentially acting protein kinases and a scaffold protein Ksr. Raf is recruited to the membrane by binding to Ras · GTP and then activated. It then phosphorylates MEK, a dual specificity kinase that phosphorylates MAP kinase. Phosphorylated MAP kinase dimerizes and translocates to the nucleus where it regulates gene expression. RTKs, GPCRs, and other receptor classes can activate MAP kinase pathways. In MAP kinase pathways containing common components, the activity of shared components is restricted to only a subset of MAP kinases by their assembly into large pathway-specific signaling complexes. Some MAP kinases have kinase-independent functions that can restrict signals to only a subset of MAP kinases. The pathway of signal transduction, Second Messengers cAMP and Other Second Messengers Activate Specific Protein Kinases 1) cAMP-dependent protein kinases (cAPKs) cAPKs Activated by Epinephrine Stimulation Regulate Glycogen Metabolism Kinase Cascades Permit Multienzyme Regulation and Amplify Hormone Signals effector enzyme adenylyl cyclase, which synthesizes the second messenger cAMP. Phosphodiesterase, PDE The cAMP-dependent protein kinases are tetramers, consisting of two regulatory (R) subunits and two catalytic (C) subunits. Binding of cAMP to the R subunits causes dissociation of the two C subunits, which then can phosphorylate specific acceptor proteins. Cellular Responses to cAMP Vary among Different Cell Types The effects of cAMP on the synthesis and degradation of glycogen are confined mainly to liver and muscle cells, which store glycogen. However, cAMP also mediates the intracellular responses of many other cells to a variety of hormones that stimulate Gs protein coupled receptors. The effects of cAMP on a given cell type depend, in part, on the specificity of the particular cAPK and on the cAPK substrates that it expresses. Anchoring Proteins Localize Effects of cAMP to Specific Subcellular Regions Modification of a Common Phospholipid Precursor Generates Several Second Messengers The inositol group in phospholipid, which extends into the cytosol adjacent to the membrane, can be [reversibly] phosphorylated at various positions by the combined actions of various kinases and phosphatases. The levels of PIs in cells are dynamically regulated by extracellular signals. In response to some signals (e.g., PDGF), there is an acute rise in PIs phosphorylated at this position through the activation of PI-3 kinase. Phosphoinositides can be cleaved by the membraneassociated enzyme phospholipase C (PLC) to generate yet other second messengers. These cleavage reactions produce 1,2-diacylglycerol (DAG), a lipophilic molecule that remains linked to the membrane, and free phosphorylated inositols, which can diffuse into the cytosol. The main pathway generates DAG and inositol 1,4,5-trisphos-phate (IP3). Signaling pathways involving any of these second messengers sometimes are referred to as inositol-lipid pathways. Hormone-Induced Release of Ca2+ from the ER Is Mediated by IP3 Opening of Ryanodine Receptors Releases Ca2+ Stores in Muscle and Nerve Cells Ca2+-Calmodulin Complex Mediates Many Cellular Responses A small cytosolic protein called calmodulin, which is ubiquitous in eukaryotic cells, mediates many cellular effects of Ca2+ ions. Ca2+-calmodulin complex is cAMP phosphodiesterase; this enzyme degrades cAMP regulation and activates several protein kinases that, in turn, phosphorylate transcription factors, thereby modifying their activity and regulating gene expression. DAG Activates Protein Kinase C, Which Regulates Many Other Proteins Synthesis of cGMP Is Induced by Both Peptide Hormones and Nitric Oxide SUMMARY Second messengers activate certain protein kinases. Phosphorylation of a specific region of the catalytic domain called the phosphorylation lip further activates these protein kinases. cAMP-dependent protein kinases (cAPKs) mediate the diverse effects of cAMP in different cells. The effect of cAMP in a cell depends largely on the particular cAPK and the protein substrates that it contains. In liver and muscle cells, hormone-induced activation of cAPK sets into motion a kinase cascade that both inhibits glycogen synthesis and stimulates glycogen breakdown. Kinase cascades triggered by cAPK and other second messenger controlled protein kinases can regulate multiple target proteins. Localization of cAPK to specific regions of the cell by anchoring proteins restricts the effects of cAMP to particular subcellular locations. Signaling through GPCRs and RTKs stimulates PI-3 kinase to generate specific phosphoinositides. Activation of both GPCRs and RTKs activate phospholipase C, which hydrolyzes PIP2 to the second messengers IP3, which diffuses into the cytosol, and DAG, which remains membrane bound. Hormone stimulation of the inositol-lipid pathway leads to the IP3mediated release of Ca2+ ions and activation of protein kinase C by DAG. Ca2+ forms a complex with a multivalent Ca2+- binding protein called calmodulin. The Ca2+-calmodulin complex regulates the activity of many different proteins, including protein kinases that in turn regulate the activity of various transcription factors. Protein kinase C is coordinately regulated by Ca2+, which recruits it to the membrane, and DAG, which activates it. cGMP is produced by cell-surface receptors with guanylate cyclase activity, which are activated by peptide hormones, and by soluble guanylate cyclase, which is activated by binding of nitric oxide. Interaction and Regulation of Signaling Pathways The Same RTK Can Be Linked to Different Signaling Pathways Multiple G Proteins Transduce Signals to Different Effector Proteins G b g Acts Directly on Some Effectors in Mammalian Cells Glycogenolysis Is Promoted by Multiple Second Messengers Molecular Mechanisms of Signal Transduction cAMP as the second messenger-which mediates the cellular response to epinephrine; other fundamental hormone mechanisms, involving different second messengers (cGMP, DAG, IP3, Ca2+), a protein-tyrosine kinase activity, and ligand- and voltage-activated ion channels; The phosphorylation and dephosphorylation of specific proteins are shown to be central to these mechanisms; steroid hormones function through the regulation of gene activity; Receptors for Epinephrine Trigger Cyclic AMP Production Cyclic AMP Acts as a Second Messenger for a Number of Regulatory Molecules Cyclic GMP Also Acts as a Second Messenger The Insulin Receptor Is a Tyrosine-Specific Protein Kinase Two Second Messengers Are Derived from Phosphatidylinositols Calcium Is a Second Messenger in Many Signal Transductions Ion Channels Are Gated by Ligands and by Membrane Potential Toxins, Oncogenes, and Tumor Promoters Interfere with Signal Transductions Steroid and Thyroid Hormones Act in the Nucleus to Change Gene Expression Insulin Stimulation Activates MAP Kinase and Protein Kinase B Ras-Dependent Pathway Ras-Independent Pathway Insulin and Glucagon Work Together to Maintain a Stable Blood Glucose Level Receptors for Many Peptide Hormones Are DownRegulated by Endocytosis Phosphorylation of Cell-Surface Receptors Modulates Their Activity Arrestins Have Two Roles in Regulating G Protein Coupled Receptors SUMMARY Many RTKs and GPCRs activate multiple signaling pathways, and different second messengers sometimes mediate the same cellular response. Some activated RTKs are coupled to the Ras-MAP kinase pathway or inositol-lipid pathway in a tissuespecific manner. Eukaryotes possess multiple G a , G b , and G g subunits. Different G a subunits activate various effector proteins, leading to production of specific second messengers . The activity of some effector proteins, including certain adenylyl cyclase isoforms, is regulated by G b g . Glycogen breakdown and synthesis is regulated by multiple second messengers induced by neural or hormonal stimulation. The insulin receptor, a dimeric RTK, can act through a Ras-dependent pathway, leading to activation of MAP kinase, or through a Rasindependent pathway involving phosphoinositides, leading to activation of protein kinase B. Insulin stimulation of muscle cells and adipocytes leads to activation of protein kinase B, which promotes glucose uptake and glycogen synthesis, resulting in a decrease in blood glucose. Binding of glucagon to its GPCR promotes glycogenolysis and an increase in blood glucose via the cAMP-triggered kinase cascade. Ligand binding frequently induces phosphorylation of the cytosolic domain of a cell-surface receptor, thereby modulating its activity. At high ligand concentration, some cell-surface receptors are internalized by endocytosis, reducing the number of receptors on the surface and making cells less sensitive to ligand. Many internalized RTKs are degraded in lysosomes. In this case, resensitization depends on synthesis of additional receptor molecules. Internalization of phosphorylated (inactive) GPCRs leads to receptor dephosphorylation, b-arrestin dissociation, and recycling of active receptors to the cell surface. From Plasma Membrane to Nucleus In one cell surface → nucleus signaling pathway, binding of interferon g to its cell-surface receptor induces membrane recruitment and activation of a cytosolic protein-tyrosine kinase called JAK. The activated JAK then phosphorylates Stat1, a member of transcription factors. Cytosolic Smad transcription factors are activated by receptor serine/threonine kinases that bind growth factors in the TGFb superfamily. The cAMP and MAP kinase pathways, activated cytosolic kinases translocate to the nucleus where they directly modify transcription factors. CREB Links cAMP Signals to Transcription In mammalian cells, an elevation in the cytosolic cAMP level stimulates the expression of many genes. cAMP-response element (CRE) binds the phosphorylated form of a transcription factor called CRE-binding (CREB) protein MAP Kinase Regulates the Activity of Many Transcription Factors Phosphorylation-Dependent Regulates NF-kB Protein Degradation NF-kB is a heterodimer of two related proteins of 65 kD and 50 kD. The proteins share a region of homology at their N-termini that is required for DNA binding and dimerization. In response to an extracellular signal, NFkB translocates to the nucleus, where it binds to specific sites in DNA and regulates transcription. NF-kB is sequestered in an inactive state in the cytoplasm by direct binding to an inhibitor called I-kB. In response to signal, I-kB is phosphorylated at two N-terminal serine residues by an I-kB kinase complex. Phosphorylated I-kB is targeted for ubiquitination and degraded in the proteosome. Phosphorylation-dependent degradation of the cyclin-kinase dependent inhibitor, Sic 1, plays a central role in regulation. It seems that phosphorylation-dependent protein degradation may emerge as a common regulatory mechanism in many different cellular processes. SUMMARY Protein phosphorylation plays a key role in regulating transcriptional activity in response to specific extracellular signals. The catalytic subunit of cAMP-dependent protein kinase translocates to the nucleus, where it phosphor-ylates CREB protein, which then interacts with the coactivator CBP/P300. The resulting trimeric complex binds to and activates transcription of target genes containing the CRE sequence. CBP/P300 also physically interacts with transcription factors whose activity is modulated by other signaling pathways. MAP kinase, activated via the RTK-Ras pathway, translocates to the nucleus, where it phosphorylates various transcriptional activators and repressors. MAP kinase phosphorylation promotes the activity of some transcription factors and inhibits the activity of others. NF-kB is localized to the cytoplasm in unstimulated cells bound to an inhibitor I-kB. In response to signal, I-kB is phosphorylated, ubiquitinated, and degraded in the proteosome. NF-kB translocates to the nucleus and regulates gene expression.