TyrPhos12
advertisement

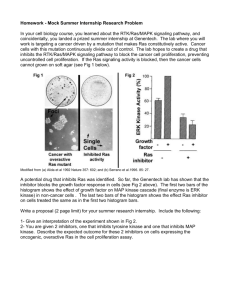
Signaling by Tyrosine Phosphorylation in the Nervous System Introduction • Protein phosphorylation represents the most common form of posttranslational modification in nature • Protein function altered by addition of a negatively charged phosphate group to a Ser, Thr, or Tyr residue: – Binding properties – Enzymatic activity if a catalytic protein Introduction • Cell surface receptors recruit activity of protein kinases in two general ways: – Non-receptor tyrosine kinases: Receptors lacking self-contained kinase function recruit activities of intracellular protein kinases to the plasma membrane – Receptor tyrosine kinases: Possess an intrinsic tyrosine kinase activity that is part of the receptor protein. Examples include receptors for growth factors (PDGF, EGF, insulin, etc.) Receptor tyrosine kinases • Introduction: – Protein phosphorylation – Recruitment of kinases in signalling pathways – Consequences of protein phosphorylation • RTK family: – Classification and structure/function – RTK ligands – Receptor dimerization and autotransphosphorylation RTK family classification and structure/function • Four common structural features shared among RTKs: – Extracellular ligand-binding domain – Single transmembrane domain – Cytoplasmic tyrosine kinase domain(s) – Regulatory domains Seven subfamilies of receptor tyrosine kinases RTK family classification and structure/function • Implicated in diverse cellular responses: – Cell division – Differentiation – Motility • At least 50 RTKs identified: – Subdivided into 10 subclasses based on differences within extracellular, ligand-binding domain of receptor • “Oncogenic” RTK mutants exist: – erbB gene encodes an N-terminal truncated, constitutively active form of EGF receptor Receptor tyrosine kinases • RTK-mediated pathways: – Ras-Raf-MAP kinase pathway, use of dominant negative mutants to map pathway – R7 photoreceptor development pathway in Drosophila RTK structure/function Regulatory domains RTK Ligands • • • • Typically small soluble proteins Work in autocrine and paracrine manner Dimerize (may aid in receptor dimerization) Some RTK ligands membrane-bound RTK Autotransphosphorylation Receptor Dimerization and Autotransphosphorylation • Ligand-induced RTK activation induces receptor dimerization, leading to activation of catalytic domains. • Receptor autotransphosphorylation: – Further stimulates kinase activity – Leads to phosphorylation of additional proteins involved in receptor signalling pathway – Provides “docking sites” for downstream signalling proteins (Grb2, PI3-kinase, phospholipase Cg, etc.) Src homology (SH)2 and SH3 domains Supposed Purpose: Joins, combines, targets kinases and phosphatases (see below) with activated (ligand-bound) growth factor receptors. SH2 and SH3 refer to domains that bind specific peptides containing a phosphorylated tyr or pro-rich sequence, respectively. Src homology (SH)2 and SH3 domains • Tyr phosphorylation allows recruitment of proteins that possess domains that bind to specific peptide sequences that encompass a phosphorylated tyr and which activating a variety of signalling pathways. • Such domains include SH2 and SH3 and P-tyr binding (PTB) domains. • These domains bind to the GF receptor only when it is phosphorylated on tyr residues. • SH2 domains: bind P-Tyr-containing sequences. • SH3 domains: bind to pro-rich (PxxP) sequences. • The proteins that contain SH2 domains belong to several categories: PLCγ, PI-3K, Grb2, SHP-2, Src (next slide): SH2 and SH3 domains RTK-mediated pathways: one pathway with two very different functions • Ras-Raf-MAP kinase pathway. • R7 photoreceptor development in Drosophila. • RTK Signaling: Ras Pathway The regulation of Ras activity, a famous downstream molecule of RTK responsible for cancer development Three ways in which signaling proteins can cross-link receptor chains 1. dimer, 2. monomer but brought together by proteoglycan, 3. cluster on membrane The importance of receptor oligomerization The docking of signaling molecules at RTK The activation of Ras by RTK signaling The MAP-kinase regulated by Ras The Ras-Raf-MAP kinase pathway SH2 domain SH3 domains Proline-rich regions (-PXXP-) GDP Tyr-P GTP Raf Grb2 SOS (inactive) Nucleus P jun Ras Pi (active) P MEK P fos Ras P P P P MAP kinase Increase gene expression DNA MAP kinase Use of oncogenic and dominant negative mutants to map pathways • Oncogenic Ras (V12Ras): defective GTPase function. Always turned “on” (always GTPbound) • Dominant negative Ras (N17Ras): can interact with its immediate upstream partner (SOS), but cannot become activated to transduce a downstream signal (i.e., to Raf). Effect is to “sequester” SOS to prevent it from activating endogenous Ras. Dominant negative Ras (N17Ras) sequesters SOS and blocks pathway from Ras on down SH2 domain SH3 domains Proline-rich regions (-PXXP-) GDP Tyr-P GDP Raf Grb2 Sos Ras (inactive) Nucleus fos N17Ras jun gene expression blocked MEK DNA MAP kinase Combine oncogenic and DN mutants to map position of pathway components SH2 domain SH3 domains Proline-rich regions (-PXXP-) GDP Tyr-P Grb2 Sos GDP Ras N17Ras Raf (inactive) Nucleus P P Oncogenic Raf P fos jun MEK P P P P MAP kinase Increased gene expression DNA MAP kinase R7 photoreceptor development • Fruitfly (Drosophila melanogaster) • Compound eye (800 ommatidia) • Each ommatidium has 8 photoreceptor cells; each detects a different wavelength of light R7 photoreceptor development • Photoreceptor cells “recruited” as an undifferentiated precursor from epithelial sheet of cells • Each photoreceptor develops in a specific order beginning with R8 and ending with R7 (responds to ultraviolet light) The R7 Photoreceptor Developmental Pathway is a RTK-MAP Kinase Cascade • RTK Signaling: • PI 3-Kinase Pathway The inositol phospholipids generated by PI3K The recruitment of signaling molecules with PH domains to the plasma membrane during B cell activation One PI3K pathway PH domain: pleckstrin homology domain Another PI3K pathway to regulate cell survival Intracellular Signaling Pathways activated by RTKs and GPCRs RTKs – Some Additional Important Points • See next slide: • Low-abundance proteins, their activation exerts major effects due to simultaneous activation of several signaling pathways that are often synergistic and enhanced survival and growth. • This is an important function of growth factors when they are located on dendritic spines or shafts or on nerve terminals. • Ser/Thr kinases can alter gene expression. • RTKs can/must be regulated (attenuated) – if not, perhaps because of some mutation, such signaling intermediates escape such control cancer. • Include mechanisms, such as desensitization, degradation, and dephosphorylation of tyrs. RTK Activation Lead to the Activation of Several Ser/Thr Kinases with a wide Variety of Substrates: CREB – end-point of several signaling pathways Non-receptor Tyrosine Kinases • Heterogeneous group of enzymes that share a common conserved tyr cat domain and a lack of extracellular ligand-bindiing domain. • At least 32 known non-receptor tyr kinases in humans distributed into 10 families. • Diverse functions • JAK/STAT-Cytokine receptors complex resemble RTKs in their ability to transduce signals in response to direct activation by extracellular signals/ligands. • Cytokine receptors are a receptor for CNTF and for leptin. Examples of Non-receptor Tyr Kinases • Conserved catalytic domains – black • Note that in JAKs, only the C-term tyr kinase domain is catalytically active. Activation Mechanism of a Cytokine Receptor coupled to JAK tyr Kinases and of STAT Cytokine, hormone… Receptor Out In JAK tyr kinase P P STAT P P STAT Nucleus P P STAT STAT STAT P P P STAT P P STAT STAT P Transcription STAT-regulated genes Activation of c-Src Myristic acid at N-term allows Covalent attachment to membrane Myristoylation enriches Src kinases in membrane rafts Two modes of intrinsic inhibition by interactions between: (1) SH2 domain and phosphorylated Y527; (2) SH3 domain and Polyproline region. Regulation of Src Family of Kinases • These kinases are attached to the membrane through an N-terminal myristic acid. • Maintained in an inactive state by intramolecular interactions that can be alleviated as indicated. • Note the unusual situation in which a tyr phosphatase can activate a tyr phosphorylation pathway. • Another way of activating Src is displacement of its SH2 domain from the C-terminal phos’d tyr by a competing phosphopeptide. Protein Tyrosine Phosphatases (PTPs) • Overall, the various types of PTPs are rather dissimilar (lacking sequence homology). • 2 Types: Receptor-like (RPTP)s and Non-receptorlike PTPs. • Among the RPTPs, there is a highly conserved cys residue in the conserved catalytic domains (some PTPs have 2 catalytic domains, although the Cterm one has little-to-no catalytic activity). • Although PTPs tend to oppose tyr kinase signalling, in some cases, they can activate specific tyr kinases (above, Src). Inactivation of MAP kinases (ERK) by threonine or tyrosine dephosphorylation Role of Protein Tyr Phosphorylation During Development of the Nervous System • Growth factors signaling. • Synaptogenesis. - NMJ – MusK (a tyr kinase, see preceding slide) and agrin AchR clustering. • Eph receptors (see slide 6) and their ligands, ephrins, participate in bidirectional signaling between cells in the travelling growth cone. Role of Tyr Phosphorylation in the Regulation of Ion Channels and Receptors NMDA R NMDA R P Tyr P-Tyr EphB2 Ca2+ PYK2/Cakβ PKC Src TrkB ? Role of Tyr Phosphorylation in Synaptic Plasticity • LTP. • Synaptogenesis: Ephrins and their receptors: In hipp cultures, Eph2 interacts with syndecan-2 (cell surface glycoprotein) to induce dendritic spine formation assoc with NMDAR clustering and synapse formation. EphB activation increases NMDAR tyr phosphorylation and glu-induced Ca2+ currents through phosphorylation by Src kinases. However, EphB2-/- appear/act normally underscoring the redundancy of multiple phos pathways in plasticity and development. Role of Tyr Phosphorylation and Phosphatases in Nervous System Diseases • Refinement of PTP chromosomal positions allows for genetic disease linkage studies: • 19 PTP chromosomal regions are frequently deleted in human cancers. • 3 PTP chromosomal regions are frequently duplicated in human cancers. • • • • • • • PTEN Tumor Suppressor Mutated in various human cancers. Cowden disease Tyr kinase Tau phosphorylation Alzheimer’s Fyn kinase Required for normal myelin formation. DEP1 Tumor suppressor Colon cancer susceptibility locus Scc1 (QTL in mice) PTPk Tumor Suppressor Primary CNS lymphomas SHP2 Noonan Syndrome Developmental disorder affecting 1:2500 newborn Stomach Ulcers Target of Helicobacter pylori • Cdc25 Cell Cycle Control primary breast cancer • PRL-3 Metastasis • FAP-1 Apoptosis Upregulated in cancers, inhibits CD95mediated apoptosis • Src Stroke Target of Myc and overexpressed in Upregulated in metastases of colon cancer Larger infarct size in mice for Src -/-