3.2 i biology notes
advertisement

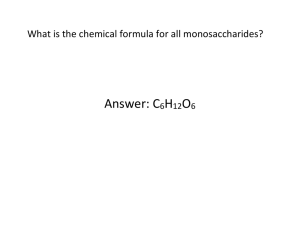
Carbohydrates Lipids Proteins Stephen Taylor Image: 'Melts In Your Hand' Found on flickrcc.net http://www.flickr.com/photos/83346641@N00/3661884940 Assessment Statements Obj. 3.2.1 Distinguish between organic and inorganic compounds. 2 3.2.2 Identify amino acids, glucose, ribose and fatty acids from diagrams showing their structure. 2 3.2.3 List three examples each of monosaccharides (glucose, galactose, fructose), disaccharides (maltose, lactose, sucrose), and polysaccharides (glycogen, cellulose, starch). 1 3.2.4 State one function of glucose, lactose and glycogen in animals and of fructose, sucrose and cellulose in plants. 1 3.2.5 Outline the role of condensation and hydrolysis in the relationships between: • monosaccharides, disaccharides and polysaccharides • fatty acids, glycerol and triglycerides • amino acids and polypeptides 2 3.2.6 State three functions of lipids, including energy storage and thermal insulation (also: protection, membranes, buoyancy, neuron insulation). 1 3.2.7 Compare the use of carbohydrates and lipids in energy storage • energy storage per gram (kJ/g) • fast/slow release of energy • demand for oxygen & ease of release of energy 3 Command terms: http://i-biology.net/ibdpbio/command-terms/ Assessment statements from: Online IB Biology Subject Guide Organic compounds contain carbon and are found in living things. They usually contain C-H or C-C bonds. The organic compounds we study can be used in metabolic reactions. Some inorganic compounds also contain carbon. A generalized amino acid The basic structure of the amino acids is common. There are 22 different protein-making amino acids, though only 20 are coded for in genetic code. Each has its own unique R-group. Some are polar, others non-polar and their different properties determine their interactions and the shape of the final protein. Amino Group (-NH2) The amino group is one of the reasons why nitrogen is an important element in living things. Carboxylic Acid Group (-COOH) The carboxylic acid group contains an oxygen double-bonded to the carbon and a hydroxyl group (-OH) that can be lost to form new bonds. Methionine: an amino acid Methionine is an important amino acid as it is coded by the START codon in mRNA (AUG). This means that is is the first amino acid in all polypeptide chains as it is the first produced in transcription in the ribosomes. Sulphur forms strong bonds (disulphide bridges) with other S-containing amino acids. Although methionine (Met) has quite a large R-group, we can still identify the amino group and carboxylic acid group on the amino acid. The simplest amino acid is glycine, with H in the R-group position. http://en.wikipedia.org/wiki/Methionine Glucose This is the basic mono-saccharide (single-unit) hexose (6-carbon) sugar molecule that is used in respiration. It is a chemical store of energy. General formula: C6H12O6 We count the carbons in clockwise direction, starting with the first carbon after the oxygen atom in the ring. Ribose This is the basic mono-saccharide (single-unit) pentose (5-carbon) sugar molecule. It is found in RNA and a similar version in DNA. General formula: C5H10O5 We count the carbons in clockwise direction, starting with the first carbon after the oxygen atom in the ring. Fatty Acids & Glycerol Fatty acid chains can be of many lengths, extended by adding CH2 units. They are an efficient store of energy and bond with glycerol (a simple sugar alcohol) to make triglycerides – lipids. http://avogadro.openmolecules.net/wiki/Main_Page Physical Modeling Which molecule is this? How do you know? Use the models to make glucose and ribose. amino acid (glycine) glucose glycerol ribose this one (glycine) Identify these organic molecules. fatty acid ribose glucose amino acid State one function of glucose, lactose and glycogen in animals, and of fructose, sucrose and cellulose in plants. Glucose is used in cell respiration to produce ATP for use in energy processed in cells. Glucose + Oxygen Carbon dioxide + water Glycogen is an insoluble storage molecule in the liver. When blood glucose is high, the pancreas releases insulin, telling the liver to capture blood glucose and combine molecules of glucose to make the polysaccharide glycogen, through condensation reactions. This stores energy for later. When blood glucose drops, the hormone glucagon causes the glycogen to be broken down (hydrolysis reactions) to glucose and then released back into the blood. Liver from: http://en.wikipedia.org/wiki/File:Leber_Schaf.jpg blood glucose too high blood glucose too low Lactose Lactose is a disaccharide produced in mammal mothers. It consists of glucose and galactose and is easily digested by the lactase enzyme in the young animal’s digestive system. By producing a small disaccharide that can be broken down by lactase, the mother can provide her young with a source of energy that can be quickly digested after feeding and then readily used in respiration. Breastfeeding logo from: http://en.wikipedia.org/wiki/File:Breastfeeding-icon-med.svg Condensation reactions make bonds. Hydrolysis bonds break these bonds. Watch these three animations and make a generalisation about the processes: - function, roles of enzymes, roles of water http://is.gd/PeptideBond http://is.gd/MaltoseGIF http://is.gd/TriglycerideGIF Animation: http://is.gd/PeptideBond Animation: http://is.gd/PeptideBond http://www.biotopics.co.uk/as/aminocon.html Carbohydrates vs Lipids for energy storage Data-based Question Emperor Penguin Masses Page 50 in the Course Companion Image source: http://www.arkive.org/emperor-penguin/aptenodytes-forsteri/image-G57959.html What are some uses of lipids in living things? For more resources. Please consider a donation to charity via Biology4Good. Click here for more information about Biology4Good charity donations. This is a Creative Commons presentation. It may be linked and embedded but not sold or re-hosted.