Specific Heat Capacity Values - Varga
advertisement
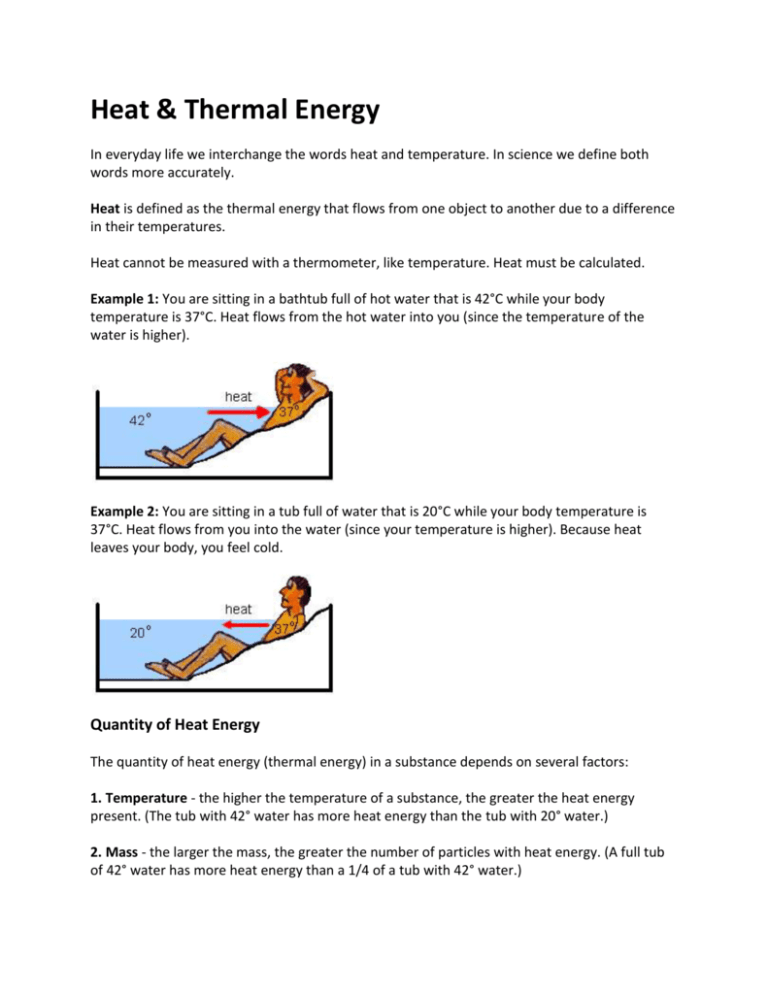
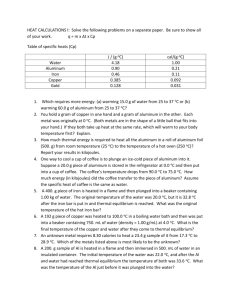
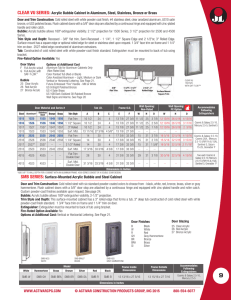
Heat & Thermal Energy In everyday life we interchange the words heat and temperature. In science we define both words more accurately. Heat is defined as the thermal energy that flows from one object to another due to a difference in their temperatures. Heat cannot be measured with a thermometer, like temperature. Heat must be calculated. Example 1: You are sitting in a bathtub full of hot water that is 42°C while your body temperature is 37°C. Heat flows from the hot water into you (since the temperature of the water is higher). Example 2: You are sitting in a tub full of water that is 20°C while your body temperature is 37°C. Heat flows from you into the water (since your temperature is higher). Because heat leaves your body, you feel cold. Quantity of Heat Energy The quantity of heat energy (thermal energy) in a substance depends on several factors: 1. Temperature - the higher the temperature of a substance, the greater the heat energy present. (The tub with 42° water has more heat energy than the tub with 20° water.) 2. Mass - the larger the mass, the greater the number of particles with heat energy. (A full tub of 42° water has more heat energy than a 1/4 of a tub with 42° water.) 3. Substance - different substances vary in their thermal properties. When you eat a hot pizza, you burn your tongue on the sauce not the crust. This thermal property is called specific heat capacity, and this also determines the amount of heat energy in a substance. Specific heat capacity is defined as the quantity of heat (energy) needed to raise the temperature of 1 kg of a substance by 1°C. Heat, like other energies, is measured in the unit, joules (J). In formula format specific heat capacity is: Example: 903 J of energy must be added to raise the temperature of 1 kg of aluminum by 1°C. Therefore the specific heat capacity of aluminum is 903 J/kg•°C. Water has a specific heat capacity of 4.2 x 103 J/kg•°C, which means that it takes 4.2 x 103 J of energy to raise 1kg of water by 1°C. Temperature can be measured using a thermometer. However, heat must be calculated using the formula: Q = mc∆T Q is the quantity of heat gained of lost, in joules m is the mass in kilograms c is the specific heat capacity in J/kg•°C ∆T is the temperature change in °C Substances with a low specific heat capacity warm quickly because they need less heat energy for a given change in temperature. They also give up their heat quickly. Substances with a high specific heat capacity take a long time to warm up and they retain their heat for a long time. Examples: How much heat is needed to raise the temperature of 2.0 kg of copper from 20.0°C to 70.0°C? 2. A 1.0 kg aluminum block has an initial temperature of 10.0°C. What will the final temperature of the aluminum block be if 3.0 x 104 J of heat is added? Specific Heat Capacity Values Substance Specific Heat Capacity J/kg°C Substance Specific Heat Capacity J/kg°C aluminum 9.0 x 102 alcohol (ethyl) 2.3 x 102 brass 3.8 x 102 alcohol (methyl) 2.5 x 102 copper 3.9 x 102 glycerine 2.4 x 102 glass (crown) 6.7 x 102 mercury 1.4 x 102 glass (pyrex) 7.8 x 102 nitrogen (liquid) 1.1 x 102 gold 1.3 x 102 water (liquid) 4.2 x 103 iron 4.5 x 102 water (ice) 2.1 x 103 lead 1.3 x 102 water (steam) 2.0 x 103 sand 8.0 x 102 air 1.0 x 103 silver 2.3 x 102 Heat Exchange & Mixtures The Principle of Heat Exchange states that when two substances at different temperatures are mixed, the amount of heat lost by the warmer substance equals the amount of heat gained by the cooler substance - assuming no heat is lost to the surroundings. Heat lost = Heat gained The heat loss from the 100g of 80°C water is gained by the 100g of 20°C water resulting in a final temperature of 50°C. Example Problem 1. A 1.0 kg brass block that is 88°C is placed in 0.44 kg of water at 6°C. The final temperature of the water and brass is 20°C. What is the specific heat capacity of the brass block? 2. A .0400 kg block of zinc at 115°C is placed in .500 kg of water at 15.0°C. If the specific heat of zinc is 388 J/kg°C, find the final temperature of the mixture. * Final temperature should be less that 115°C and higher than 15.0°C. Remember that ∆T should be a positive number since we take the absolute value. We can write ∆T for zinc as (115°C - Tfinal) and ∆T for water as (Tfinal - 15°C) which will give us positive values for both.