Subshells and Orbitals
advertisement

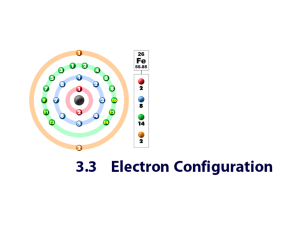
Subshells and Orbitals Timberlake LecturePLUS 2000 Quantum Mechanics Describes the arrangement of electrons in atoms in terms of: Main or principal energy levels (n) Energy subshells Orbitals (space occupied within the atom) Timberlake LecturePLUS 2000 Principal Energy Levels (n) Contain electrons that are Close in energy Similar distance from nucleus Have values of n = 1, 2, 3, 4, 5, 6….. Maximum number of electrons = 2n2 n =1 n =2 n=3 2(1)2 = 2 2(2)2 =8 Timberlake LecturePLUS 2000 Energy Levels (Shells) • A group of electrons in an atom all having the same principal quantum number (n) n = 1, 2, 3, … • The first shell (n = 1) is lowest in energy, 2nd level next and so on 1<2<3<4 • The number of electron in each shell is limited to 2n2 n=1 n=2 2n2 = 2 2 Timberlake LecturePLUS 2000 2n = ____ Energy Levels for Electrons Some possible electron transitions for the first three energy levels are shown below. The negative value means that the electron in the atom has a lower energy than a free electron Energy Level Energy, E n=3 ___________________ (-) 2.420 x 1019 J n=2 __________________ (-) 5.445 x 1019 J 18 J n=1 __________________ (-) 2.178 x 10 Timberlake LecturePLUS 2000 Learning Check S1 A. What energy change (J) takes place when an electron in a hydrogen atom moves from the first (n=1) to the second shell (n=2)? B. What energy change (J) takes place when the electron moves from the third shell to the second shell? Timberlake LecturePLUS 2000 Solution S1 A. What energy change takes place when an electron in a hydrogen atom moves from the first (n=1) to the second shell (n=2)? 1.634 x 10-18 J of energy must be absorbed. B. What energy change takes place when the electron moves from the third shell to the second shell? (-5.445 x 10-19J ) - (2.2420 x 10-19 J) = -3.025 x 1019J will be emitted as electron falls from a higher to a lower energy state Timberlake LecturePLUS 2000 Subshells Energy sublevels within energy level All electrons in a subshell have the same energy Designated s, p, d, f .. Sublevel energy: s<p<d<f Timberlake LecturePLUS 2000 Electron Locations Main Energy Levels n=4 Sublevels 4s, 4p, 4d, 4f n=3 3s, 3p, 3d n=2 2s, 2p n=1 1s Timberlake LecturePLUS 2000 Sublevels in n = 1,2, 3 n=3 3d 3p 3s n=2 2p 2s n=1 1s Timberlake LecturePLUS 2000 Electrons Allowed All electrons in the same sublevel have the same energy. All 2s electrons have the same energy. All 2p electrons have the same energy which is slightly higher than the energy of the 2s electrons s sublevel 2 electrons p sublevel 6 electrons d sublevel 10 electrons f sublevel 14 electrons Timberlake LecturePLUS 2000 Electron Configuration List of subshells containing electrons Written in order of increasing energy Superscripts give the number of electrons Example: Electron configuration of neon number of electrons 1s2 main shell 2s2 2p6 subshell Timberlake LecturePLUS 2000 Order of Filling Total energy of a subshell = energy of the main shell + the subshell The 4s energy < 3d energy 4p ___ 3d ___ (finishes the n=3 shell) 4s ___ (starts the n=4 shell) 3p ___ 3s ___ 2p ___ 2s ___ 1s ___ Timberlake LecturePLUS 2000 Writing Electron Configurations H 1s1 He 1s2 Li 1s2 2s1 C 1s2 2s2 2p2 S 1s2 2s2 2p6 3s2 Timberlake LecturePLUS 2000 3p4 Sublevel Blocks s1 s2 1 2 3 4 5 6 p1 p2 p3 p4 p5 p6 d1 - d10 f1 - f14 Timberlake LecturePLUS 2000 Periodic Table and Electron Configuration Find the element on the periodic table Use the order of filling indicated across each period Groups 1-2 = s level Groups 3-8 = p level Transition = d level Lantanides = f level Timberlake LecturePLUS 2000 Learning Check S2 Indicate if each configuration is (1) correct or (2) incorrect for potassium. Give an explanation for selection of 1 or 2. Explain why or why not? A. 1s22s22p63s1 1 or 2 B. 1s22s22p63s23p6 1 or 2 C. 1s22s22p63s23p64s1 1 or 2 D. 1s22p83s1 1 or 2 E. 63s23p7 1s22s22p Timberlake LecturePLUS 2000 1 or 2 Solution E2 For phosphorus, indicate if each configuration is (1) correct or (2) incorrect. Explain why or why not. A. 2, 2, 8, 5 2 B. 2, 8, 3 2 C. 2, 8, 5 1 D. 2, 6, 7 2 Timberlake LecturePLUS 2000 Learning Check S3 Using the periodic table, write the complete electronic configuration for each: A. Cl B. Sr C. I Timberlake LecturePLUS 2000 Solution S3 Using the periodic table, write the complete electronic configuration for each: A. Cl 1s2 2s2 2p6 3s2 3p5 B. Sr 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 C. I 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5 Timberlake LecturePLUS 2000 Learning Check S4 A. The final two notations for Co are 1) 3p64s2 2) 4s24d7 3) 4s23d7 B. The final three notations for Sn are 1) 5s25p24d10 2) 5s24d105p2 3) 5s25d105p2 Timberlake LecturePLUS 2000 Solution S4 A. The final two notations for Co are 3) 4s2 3d7 B. The final three notations for Sn are 2) 5s2 4d10 5p2 Timberlake LecturePLUS 2000 Orbital A 3 dimensional space around a nucleus in which electrons are most likely to be found Shape represents electron density (not a path the electron follows) Each orbital can hold up to 2 electrons. Timberlake LecturePLUS 2000 s orbitals 1s 2s Timberlake LecturePLUS 2000 3s Three p Orbitals px pz Timberlake LecturePLUS 2000 py p subshell contains p orbitals Timberlake LecturePLUS 2000 d orbitals Timberlake LecturePLUS 2000 Learning Check S5 A. Number of electrons in a p orbital 1) 1e 2) 1e or 2e 3) 3e B. Number of orbitals in a p subshell 1) 1 2) 2 3) 3 C. Number of orbitals in 4d subshell 1) 1 2) 3 3) 5 D. Number of electrons (maximum) in a 3d subshell 1) 2e 2) 5e 3) 10e Timberlake LecturePLUS 2000 Solution S5 A. Number of electrons in a p orbital 2) 1e or 2e B. Number of orbitals in a p subshell 3) 3 C. Number of orbitals in 4d subshell 3) 5 D. Number of electrons in a 3d subshell 3) 10e Timberlake LecturePLUS 2000