inflammation
advertisement

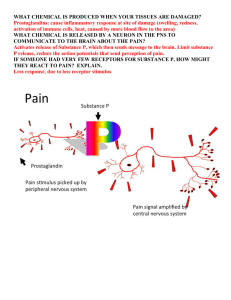
PHARMACOLOGY OF INFLAMMATION David J. Mokler, Ph.D. October 29, 2009 OBJECTIVES After studying this material the student should; Describe the role of prostaglandins in the inflammatory response. Describe the mechanism of action of non-steroidal drugs used in the treatment of inflammation. Describe the pharmacology and toxicology of the non-steroidal anti-inflammatory agents. Discuss the toxicity of acetaminophen OBJECTIVES After studying this material the student should; Discuss the proper and rational use of corticosteroids in the treatment of inflammation. Discuss the toxic effects of the corticosteroids used in chronic therapy. NSAIDs – COX INHIBITORS Aspirin Indomethacin (INDOCIN) Proprionic Acid Derivatives Ibuprofen (MOTRIN, RUFEN, ADVIL, NUPRIN) Naproxen (NAPROSYN) Piroxicam (FELDENE) Nabumetone (RELAFEN) Ketorolac (TORADOL) Acetominophen (TYLENOL, TEMPRA, others) NSAIDs – COX INHIBITORS COX2 Inhibitors Celecoxib (CELEBREX) PROSTAGLANDINS History 1930 - Kurzrok and Lieb 1930s - Goldblatt, Euler "prostaglandin"- lipid- soluble acid 1962 - isolation of PGE1 and PGF1 1964 - synthesis from arachadonic acid Arachadonic acid metabolites via cyclooxygenase Released by mechanical, thermal, chemical, bacterial and other trauma PROSTAGLANDINS PROSTAGLANDINS Effects produce blood flow in injured region, vascular permeability, enhance lymphocytic infiltration may modulate release of histamine from mast cells - PGD2 stimulates, PGE2 and PGI2 inhibits oxygen radicals produced as byproduct of synthesis ® inflammation potentiate pain-producing effects of kinins produce fever in hypothalamus PGE1 and PGE2 stimulate osteoclasts ® bone resorbtion Fever PROSTAGLANDINS Effects PGE1 and PGD2 inhibit platelet aggregation TXA2 induces aggregation PGA2, PGE1 and PGE2 induce erythroporesis PGFs contract, PGEs relax bronchial smooth muscle, non-pregnant and pregnant uterus Two Forms of Cyclo-oxygenase (COX) COX-1 COX-2 Produces prostanoids that mediate homeostatic functions Produces prostanoids that mediate inflammation, pain, and fever Constitutively expressed Induced mainly at sites of inflammation by cytokines Especially important in: – Gastric mucosa; smalland large-bowel mucosa – Kidney – Platelets – Vascular endothelium DuBois RN, et al. FASEB J. 1998;12:1063–1073. Constitutive expression in: – Brain – Kidney (mainly animal data) – Female reproductive tract Mechanism of Action of Anti-inflammatory Agents Arachidonic acid COX-2–targeted agents X COX-1 COX-2 X Prostaglandins Protect gastroduodenal mucosa Traditional NSAIDs X Thromboxane Prostaglandins Supports platelet function Mediate inflammation, pain, and fever LEUKOTRIENES arachidonic acid metabolites from lipoxygenase may be inhibited by some NSAIDs, inhibited by steroids LEUKOTRIENES LEUKOTRIENES Effects LTB4 potent chemotaxic substance act on endothelium of postcapillary venules to cause exudation of plasma 5-HPETE and 5-HETE induce release of histamine from basophils LTC4 and LTD4 potent bronchoconstrictors LTD4 is the slow reacting substance of anaphylaxis (SRSA) ® bronchoconstriction, histamine release, vasopermeability OTHER MEDIATORS OF INFLAMMATION Histamine 5-hydroxytryptamine (Serotonin) Bradykinins Vasopermeability histamine release prostaglandin synthesis Pain NSAIDs SALICYLATES Aspirin – acetylsalicylic acid (ASA) NSAIDs ASPIRIN (ASA) Mechanism of action inhibits prostaglandin synthesis by acetylation of cyclooxygenase Pharmacological actions Analgesia Anti-inflammatory Antipyretic action NSAIDs SALICYLATES Pharmacokinetics rapid absorption- low pH increases absorption- high pH increases solubility, enhances absorption peak blood levels: 2 hours up to 90% protein bound in plasma metabolism in liver-glycine or glucuronide conjugates; 10% is free salicylate excretion in urine free salicylate excretion (not metabolites) may be enhanced by making the urine more alkaline NSAIDs SALICYLATES Toxicity large doses will increase depth of respiration nausea and vomiting salicylism - chronic treatment of high doses- confusion-delerium- tinnitus – dizziness increased bleeding time gastric ulceration (especially with alcohol) hemorrhage may alter uric acid excretion (dose dependent) Toxicity Overdose acid-base changes - usually acidosis, metabolic & respiratory High doses suppress respiration → respiratory acidosis Uncouple oxidative phosphorylation in cells → metabolic acidosis PROPRIONIC ACID DERIVATIVES Ibuprofen (MOTRIN, ADVIL, NUPRIN) rapidly absorbed after oral administration, peak concentration 1 to 2 hours extensively (99%) protein bound, 90% excreted as metabolites in urine Naproxen (NAPROSYN) longer half life, therefore 2 x day dosing inhibits PMN migration Piroxicam (FELDENE) Nabumetone (RELAFEN) PROPRIONIC ACID DERIVATIVES anti-inflammatory, anti-pyretic, analgesic inhibit cyclooxygenase, inhibit leukocyte migration possibly by inhibition of lipoxygenase Better anti-inflammatory than aspirin? PROPRIONIC ACID DERIVATIVES Toxicity – this relates to all NSAIDs Increased bleeding time Gastric bleeds Long term may cause liver toxicity Hypertension and renal failure especially in the elderly Edema CNS – dizziness, confusion, drowsiness, anxiety Ketorolac (TORADOL) High potency, analgesia equivalent to morphine Used for moderate to severe pain Not for mild pain Short term peri-operative use Similar side effects to other NSAIDs Indomethecin (INDOCIN) Potent inhibitor of cyclooxygenase, inhibits PMN migration Analgesic, anti-inflammatory and anti-pyretic - similar to aspirin Rapidly and completely absorbed following oral administration, 90% protein bound, low concentrations in CSF but plasma levels in synovial fluid Toxicity - 35 to 50% of patients receiving therapeutic doses report side effects: GI - anorexia, nausea, abdominal pain, ulceration of upper GI tract: CNS - severe frontal headache most common (25-50%), dizziness, vertigo, light-headedness, mental confusion: hematopoietic reactions, hypersensitivity (cross-sensitivity with aspirin) Mean Plasma half-lives of different NSAIDS Drug Half-life (hr) Short Aspirin 0.25 Diclofenac 1.1 Etolodac 3.0 Ibuprofen 2.1 Indomethacin 4.6 Ketoprofen 1.8 Long Diflunisal Naproxen Phenylbutazone Piroxicam Sulindac 15 14 68 57 14 Acetaminophen (TYLENOL) Actions Analgesic, antipyretic properties comparable to salicylates Weak inhibitor of prostaglandin biosynthesis in periphery, more activity in CNS Identification in 2002 of COX-3 in brain that has a higher affinity for acetaminophen – now thought to be a splice variant of COX-1 Weak anti-inflammatory action No effect on respiration No effect on platelets No effect on uric acid excretion Acetaminophen (TYLENOL) Pharmokinetics rapid absorption peak blood levels: 1-2 hours acetaminophen to glucuronide conjugation excreted in urine Acetaminophen (TYLENOL) Toxicity Allergic reactions (rare) Toxicity at therapeutic doses 2 extra strengths = 1000 mg FDA panel recommends no more than 650 mg per dose Recommends total daily dose less than 4000 mg Evidence of long term hepatic toxicity with long term use Use of acetaminophen in many combination products Acetaminophen (TYLENOL) Toxicity in overdose No acute signs but medical emergency Hepatic necrosis, renal tubular necrosis emerges over days Hypoglycemic coma Treatment acetylcysteine COX2 inhibitor Celecoxib (CELBREX) Selectively inhibit COX2 – inducible cyclo-oxygenase May be no better than non-selective inhibitors Gastrointestinal Toxicity With Celecoxib vs Nonsteroidal Anti-inflammatory Drugs for Osteoarthritis and Rheumatoid Arthritis The CLASS Study: A Randomized Controlled Trial JAMA 284(10): 1247, 2000 http://jama.amaassn.org/issues/v284n10/rfull/joc01227.html Figure 2. Annualized Incidence of Upper Gastrointestinal Tract Ulcer Complications Alone and With Symptomatic Gastroduodenal Ulcers Figure 3. Patients With Decreases in Hematocrit and/or Hemoglobin at 6 Months Figure 4. Patients With Increases in Serum Creatinine and/or Serum Urea Nitrogen and With Elevations in ALT and AST at 6 Months What happened to the COX-2 inhibitors?? Rofecoxib (VIOXX) withdrawn In APPROVe trial Designed to evaluate the efficacy of rofecoxib, 25 mg, in preventing recurrence of colorectal polyps in 2,600 patients with a history of colorectal adenomas The increased cardiovascular risk began after 18 months of treatment with rofecoxib and persisted. At three years, cumulative incidence of cardiovascular events was 7.5 per 1,000 patients receiving placebo compared with 15 per 1,000 patients receiving rofecoxib Mechanisms of cardiovascular toxicity COX-1 helps promote thrombosis and COX-2 helps inhibit it, blocking COX2 but not COX-1 could theoretically increase the risk of myocardial infarction and other thrombotic events. Mechanisms of cardiovascular toxicity (con’t) Depression of prostaglandin I2 formation by coxibs might be expected to elevate blood pressure, accelerate atherogenesis, and increase the thrombotic response to rupture of an atherosclerotic plaque. In patients at higher cardiovascular risk, coxibs would be more likely to predispose to a clinical event early in the course of treatment CONSIDERATIONS OF THERAPY WITH NSAIDs Most NSAIDs are similar in efficacy Patient variability in efficacy and toxicity Classification by duration of action Side effects serve as the basis for therapeutic choice Inhibition of cyclooxygenase varies as to distribution in body fluids Aspirin and para-aminophenol toxicity Blood dyscrasias TREATMENT OF ARTHRITIS WITH NSAIDs Reduced inflammation slows progress of disease High dose therapy Aged population monitor renal function monitor blood for dyscrasias Use best tolerated agent and lowest cost ANTI-INFLAMMATORY STEROIDS David J. Mokler, Ph.D. October 29, 2009 CORTISOL Chemistry & Metabolism major glucocorticoid in humans synthesized from cholesterol in cells of the zona fasiculata and zona reticularis of the adrenal cortex released under the influence of ACTH 20 mg secreted per day in adult in the absence of stress 95% bound in blood to corticosteroid binding globulin T½ 90-110 min; increased with large amounts or hypothyroidism reduced and conjugated in liver, excreted in urine as 11-oxy 17-ketosteroids Physiologic and Pharmacological Effects Widespread effects - homeostasis Dose-related and "permissive" effects Effects on metabolism Protects glucose-dependent tissue (brain and heart) In periphery decreases glucose utilization Increases blood glucose Stimulates gluconeogenesis glycogen stores Anti-insulin effects Physiologic and Pharmacological Effects Cardiovascular decrease capillary permeability, incr in Na+ retention Blood elements ↑ hemoglobin and red blood cells, ↑ PMN leukocytes ↓ lymphocytes, eosinophils, monocytes, basophils ↓ lymphoid tissue and immune response Mechanisms of Action Glucocorticoid Receptor Signaling SIGMA-ALDRICH STEROIDS FOR THOUGHT Anti-Inflammatory Properties Inhibit early phase (edema, fibrin deposition, capillary dilatation, migration of leukocytes and phagocytic activity) Inhibit late phase (capillary proliferation, fibroblast proliferation, deposition of collagen and cicatrization) Inhibit inflammatory response regardless of inciting agent - palliative therapy Inhibit recruitment of neutrophils and monocyte - macrophages SYNTHETIC CORTICOSTEROIDS Structure for ↑ glucocorticoid activity 1, 2 double bond - prednisone, prednisolone - enhances glucocorticoid effects 6α -methylation - unpredictable - 6α- methyl-prednisolo ne - increased anti-inflammatory 9-fluoridation - increases all activity - paramethasone, betamethasone, dexamethasone 16-methylation eliminates Na+ retaining effect with slight change in metabolic and anti-inflammatory effect 17 α-hydroxy - maximizes carbohydrate and anti-inflammatory potency SAR and parallel activity of glucose metabolic and anti-inflammatory activity suggests similar receptor mechanisms Relative Potencies Anti-inflammatory Cortisol 1 Prednisone 4 (Δ1-Cortisone) Prednisolone 4 Fludrocortisone 10 Corticosterone 0.35 Triamcinalone 5 (9α-Fluoro-16α-hydroprednisolone) Paramethasone 10 (6α-Fluoro-16α-methylprednisolone) Betamethasone 25 (9α-Fluoro-16β-methylprednisolone) Dexamethasone 25 (9α-Fluoro-16α-methylprednisolone) Na+ 1 0.8 0.8 125 15 0 0 0 0 PHARMACOKINETICS Well absorbed after oral administration Also available for i.v, i.m., intrasynovial and topical administration 90% protein bound to corticosteroid-binding globulin - increases during pregnancy and administration of estrogens and cortisol Hepatic and extra-hepatic metabolism TOXICITY OF STEROIDS Acute and Subacute days or a few weeks few adverse effects behavioral changes acute peptic ulcers TOXICITY OF STEROIDS Chronic Therapy – Withdrawal acute adrenal insufficiency withdrawal syndrome - fever, myalgia, arthralgia, malaise Chronic Therapy - Continued High Dose Therapy Adrenal suppression supplementary therapy at times of severe stress accidental trauma, surgery recovery following withdrawal, may take 6-9 months following chronic treatment TOXICITY OF STEROIDS Chronic Therapy - High Dose Therapy Iatrogenic Cushing's syndrome redistribution of body fat from extremities to trunk and face striae, ecchymoses, acne and hirsutism Increased susceptibility to infection Nonspecific aggressive treatment with drugs specific for pathogen Hypokalemia, hypochloremia, edema - not seen with 16-substituted compounds Behavioral disturbances, psychoses - nervousness, insomnia, changes in mood, manic-depressive or schizophrenic symptoms, suicidal tendencies TOXICITY OF STEROIDS Chronic Therapy - High Dose Therapy Cataracts Osteoporosis in all ages inhibition of osteoblasts and Ca++ uptake, secretion PTH (+) osteoclasts, therefore formation and resorbtion indication for withdrawal from therapy Growth retardation in children - not reversible by exogenous HGH THERAPEUTIC USES Substitution therapy acute - cortisol in dose equivalent to maximum daily rate of secretion in stress - with intravenous isotonic saline chronic - cortisol in twice daily dosing to mimic diurnal cycle - with mineralocorticoid Arthritis rheumatoid lowest dose to alleviate symptoms continue NSAIDs, rest, physical therapy osteoarthritis - intra-articular injection controversial THERAPEUTIC USES Rheumatic carditis - non-responsive to salicylates -in combination with salicylates Renal diseases - in some cases of acute and chronic glomerulonephritis with nephrotic syndrome Collagen diseases - most diseases associated with collagen except scleroderma - life threatening, fulminating SLE Allergic disorders - manifestations of short duration controlled - used in combination with other agents - not effective for severe acute reactions - Why? THERAPEUTIC USES Ocular diseases - contraindicated in herpes simplex, mechanical lacerations, or fungal, viral or bacterial infections Skin diseases - topical steroids - psoriasis, vitiligo, seborrhea Liver diseases - only certain patients with chronic active hepatitis Cerebral edema - neoplasia, no strong evidence for value in edema due to trauma, cerebrovascular accident or shock THERAPEUTIC CONSIDERATIONS Empirical use - 6 principles Careful titration of dose A single dose of steroid, even large, has very little harmful effect A few days of therapy at moderate doses and in the absence of contraindications is unlikely to produce harmful effects Increased toxicity with increased dose and increased duration of therapy Not curative except for adrenal insufficiency Abrupt withdrawal following high-dose therapy may be life-threatening