7.6 Enzymes - HS Biology IB
advertisement

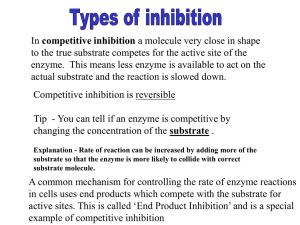
7.6 Enzymes 7.6.1: STATE: metabolic pathways consist of chains and cycles of enzyme catalysed reactions 7.6.2: Describe the induced-fit model. 7.6.3: Explain that enzymes are biological catalysts that lower the activation energy of reactions. IB Question: (i) The graph below shows the energy changes in a reaction. N07/4/BIOLO/HP2/ENG/TZ0/XX+ On the above graph draw the result you would obtain in this same reaction if an enzyme that catalyses this reaction were added. [1] (ii) Explain how the enzyme produces this effect. [3] IB Question: Outline how enzymes catalyse reactions. [7] IB Question: Outline how enzymes catalyse reactions. [7] they increase rate of (chemical) reaction; remains unused/unchanged at the end of the reaction; lower activation energy; activation energy is energy needed to overcome energy barrier that prevents reaction; annotated graph showing reaction with and without enzyme; substrate joins with enzyme at active site; to form enzyme-substrate complex; active site/enzyme (usually) specific for a particular substrate; enzyme binding with substrate brings reactants closer together to facilitate chemical reactions (such as electron transfer); induced fit model / change in enzyme conformation (when enzyme-substrate/ES complex forms); making the substrate more reactive; 7.6.4: Explain the difference between competitive and non-competitive inhibition, with reference to one example of each. E.g. Prontosil is a competitive inhibitor that is used as an antibiotic because it inhibits folic acid synthesis in bacteria. Non-competitive inhibitor. E.g.Nerve gases like Sarin is a non-competitive inhibitor function by inactivating the enzyme ethanoyl (acetyl) cholinesterase. IB Question: Compare competitive inhibition and non-competitive inhibition. [2] IB Question: Compare competitive inhibition and non-competitive inhibition. [2] N08/4/BIOLO/HP2/ENG/TZ0/XX+ IB Question: Using a table, compare competitive and non-competitive inhibition and give one named example of each. [5] IB Question: Using a table, compare competitive and non-competitive inhibition and give one named example of each. [5] 7.6.5: Explain the control of metabolic pathways by end-product inhibition, including the role of allosteric sites. 7.6.5 IB Question: Explain how end product inhibition is used to control the rate of chemical reactions in cells. Include an example in your answer with the names of both the enzyme and the inhibitor. [8] IB Question: Explain how end product inhibition is used to control the rate of chemical reactions in cells. Include an example in your answer with the names of both the enzyme and the inhibitor. [8] inhibitor binds to enzyme; inhibitor is non-competitive; binds at an allosteric site / away from the active site; shape of active site / structure of enzyme altered / conformational change; substrate cannot bind / reaction cannot be catalyzed; early / first enzyme in a pathway is inhibited by product of last reaction; the higher the end product concentration the higher the inhibition; avoids a build-up of all the intermediates; reversible / inhibitor can detach pathway restarted when there is a shortage of the end product; named inhibitor e.g. ATP / other example; named enzyme e.g. phosphofructokinase / other example; [8 max]