Writing Chemical Formulas Chapter 7 p. 203
advertisement

You will learn:
to write and read 4 types of chemical formulas
1.
2.
3.
4.
Ionic Compounds
Polyatomic Compounds
Molecular Covalent Compounds
Acids/ Bases
Writing Chemical Formulas
When 2 or more elements are chemically combined they are
called a molecule or compound.
H 2 + O2
H2O
Na + Cl
NaCl
atom is the smallest whole unit of an
element…….
A molecule is the smallest whole unit of a
compound……..
Regardless of where or how a pure chemical
compound is prepared, it is composed of a
fixed proportion of elements.
“If it is water
it is always 2 hydrogen for every one oxygen… H2O
If it is carbon dioxide
it is always one carbon for every 2 oxygen…CO2…
**Law of Definite Proportions**
• Remember:
– Valence Outer shell electrons involved in chemical reactions
– Octet Rule: Most stable condition is 8 electrons in outer shell
– Oxidation #: a + or – number which tells how many electrons
were lost
gained
shared when bonding atoms.
Label empty periodic chart
Writing Ionic Binary Compounds
(metal + nonmetal) ws 7.1
1.
Write + oxidation number atom first
2.
Write – oxidation number atom second
3.
Do cross-over method for subscripts
4.
The compound is in a neutral or “ground state”.
The formula’s oxidation #’s must add up to equal zero.
5.
Must have lowest common factor
Na + N
Ca + P
Ba + F
Mg + O
Ba + Sb
Li + S
Ca + O
Sc + Al
Naming Binary Ionic Compounds
(Stock System)
ws 7.2
1. Write first element name
2. Drop last syllable of second element and add ide
3. Transition metals use Roman Numerals to show
oxidation #
• LiF
CuCl
NaBr
CuCl2
KI
FeO
Ternary Compounds
Polyatomic Ions
Poly = many atoms in a group
When writing these formulas use the
group as a whole.
Ca + ClO3
Na + SO4
NH4 + PO4
No need to use ( ) if only one group
7.3, 7.4
worksheet
Naming Molcular Compounds
1. Nonmetals only
2. Prefixes give the number of each element
( show numbers)
3. Second element ends in ide
4. O or A at end of prefix is dropped if element
begins with a vowel.
{ monoxide … not---monooxide}
{pentoxide … not– pentaoxide}
P4S5 tetraphosphorus pentasulfide
Si2Br6 disilicon hexabromide
CH4
carbon tetrahydride
ws-7.7
Writing Formulas for Molecular
Covalent Compounds NM + NM
• Follow wording… do NOT figure oxidation #
Antimony tribromide
Hexaboron monosilicide
Chlorine dioxide
ws 7.8
formulas for Molecular Covalent = NM+Nm
1. Make sure Nonmetals + Nonmetals
2. The less electronegative element is written first
3.
4.
5.
6.
The element written second will keep its normal negative oxidation #.
The first element will keep its normal oxidation # but will be positive.
Use the cross-over method
The algebraic sum of the ox# must equal zero
1.
2.
3.
4.
5.
6.
make sure NM + NM
The less electronegative element is written first
The element written second will keep its normal negative oxidation #.
The first element will keep its normal oxidation # but will be positive.
Use the cross-over method
The algebraic sum of the ox# must equal zero
Nitrogen + fluorine
Oxygen + sulfur
Phosphrous + chlorine
Bromine + carbon
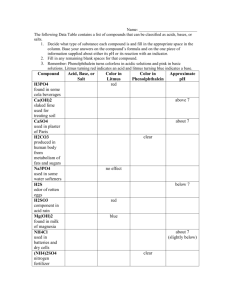
Naming Acids
2 common types of Acids
Binary Acids
Hydrogen + one other element
Hydro + root name & ic
HBr
HCl
H2S
HCN
hydrobromic acid
hydrochloric acid
hydrosulfuric acid
hydrocyanic acid
Oxyacids
hydrogen + Polyatomic
w/Oxygen
Root name + Suffix + acid
If polyatomic ended in ‘ate’
(NO3)- nitrate ion then
HNO3 = nitric acid
(SO4)-2 sulfate ion then
H2SO4 = sulfuric acid
“notice hydrogen not part of name”
It is important to remember that these hydrogen – containing compounds
are named as acids only when they are in water solutions.
Example:
HCl----hydrogen chloride is a gas.
But dissolved in water it is hydrochloric acid.
Memorize:
HCl
hydrochloric acid
HNO3 nitric acid
H2SO4 sulfuric acid
HC2H3O2
H2CO3
H3PO4
acetic acid
carbonic acid
phosphoric acid
We will learn more acids and bases in chapter 17 later
I would put these on an index card
Acids and Bases
Six Acids to memorize
Hydrochloric
Nitric
Sulfuric
Phosphoric
Carbonic
Acetic
Bases:
metal + hydroxide
Sodium hydroxide
Potassium hydroxide
Calcium hydroxide
4 ways to name chemicals
1st step----make sure you know
which method
M+NM
NM+NM
Ternary Compounds
Acids
Nameing Oxyanions