d-calorimetry-2013
advertisement
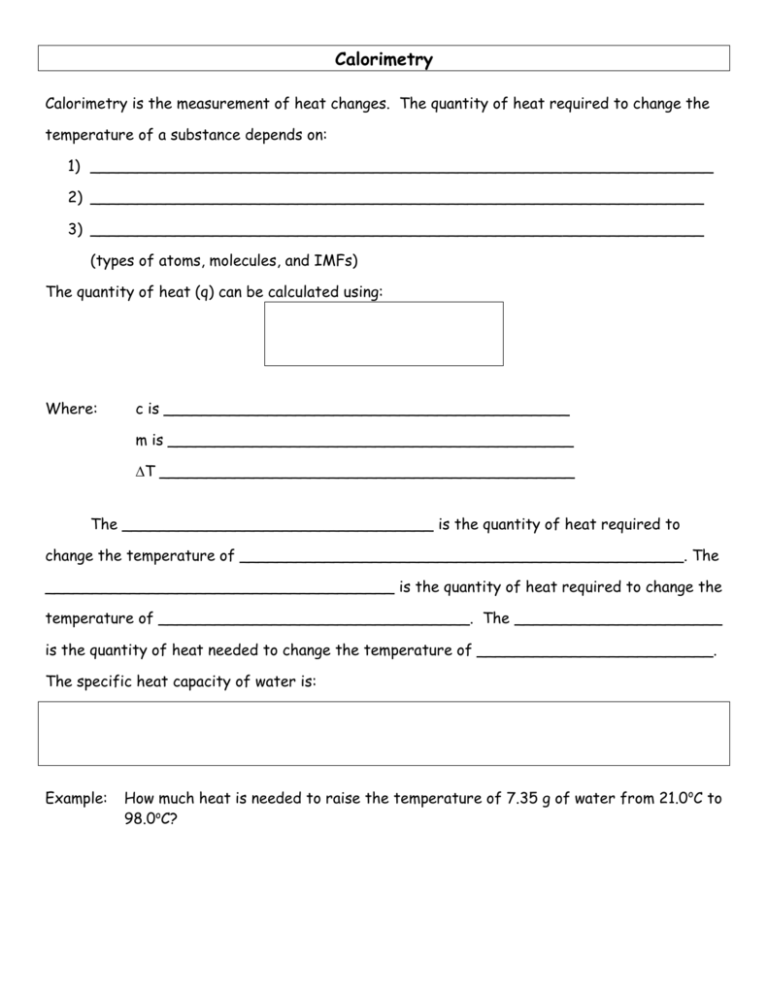
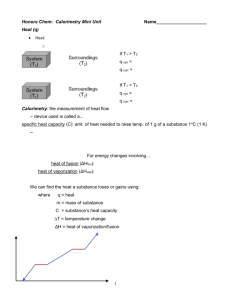
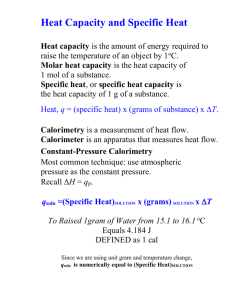
Calorimetry Calorimetry is the measurement of heat changes. The quantity of heat required to change the temperature of a substance depends on: 1) __________________________________________________________________ 2) _________________________________________________________________ 3) _________________________________________________________________ (types of atoms, molecules, and IMFs) The quantity of heat (q) can be calculated using: Where: c is ___________________________________________ m is ___________________________________________ T ____________________________________________ The _________________________________ is the quantity of heat required to change the temperature of _______________________________________________. The _____________________________________ is the quantity of heat required to change the temperature of _________________________________. The ______________________ is the quantity of heat needed to change the temperature of _________________________. The specific heat capacity of water is: Example: How much heat is needed to raise the temperature of 7.35 g of water from 21.0oC to 98.0oC? Example: Calculate the enthalpy change for the process in which 50.0 g of water is converted from liquid at 10.0oC to vapour at 25oC. (see last days notes for Hvap) Because of the First Law of Thermodynamics, the heat _______________ by a system must be _______________ the heat lost from its _______________________ and vice versa. This is a useful relationship for determining the _______________________________ of a substance. For example:A 150.0 g sample of lead at 100.0oC is placed in 50.0 g of water at 22.0oC in an insulated container. The final temperature of the lead-water mix is 28.8oC. What is the specific heat capacity of lead? The amount of heat absorbed or released during a chemical reaction can be measured with a ______________________. There are two main types of calorimeters: the ________________ calorimeter and the ___________________ calorimeter. Only coffeecup calorimeters are used in this course. The Coffee-cup Calorimeter The coffee-cup calorimeter is used for reactions _____________________________________________ in general laboratory situations. In this case the entire coffee cup and its contents are the __________________ and it has a constant ____________________ (so you are determining qp or H). To determine H you measure the change in temperature of the ______________________ as the reaction proceeds and then relate it to the reaction using ________________________________________. Example: 100.0 mL of 1.00 M HCl and 100.0 mL of 1.00 M NaOH both initially at 21.1oC are added to a coffee-cup calorimeter and allowed to react. The temperature rises to 27.8oC. Determine the heat of neutralization for this reaction in kJ/mole H2O formed. Is the reaction endo or exothermic? (Ignore the small heat capacity of the stryofoam cup and assume the only thing in the system absorbing heat is the 200 mL of solution which is almost all H2O.) Example: 50.0 mL of 0.500 M HCl and 25.0 mL of 0.500 M NaOH both initially at 25.0oC are added to a coffee-cup calorimeter and allowed to react. The temperature rises to 27.21oC. Calculate qsoln in J and Hrxn in kJ/mole H2O formed. Is the reaction endo or exothermic? (Ignore the small heat capacity of the stryofoam cup and assume the final solution has the same heat capacity and density as water.) Calorimetry can be used to determine heat capacities, enthalpies of vaporization, enthalpies of fusion, and enthalpies of reactions. Silberberg: Rd p246-249 and do Q #29, 31, 33, 39, 41, 44, 46