Metric System Units - Mr-Watson-General
advertisement
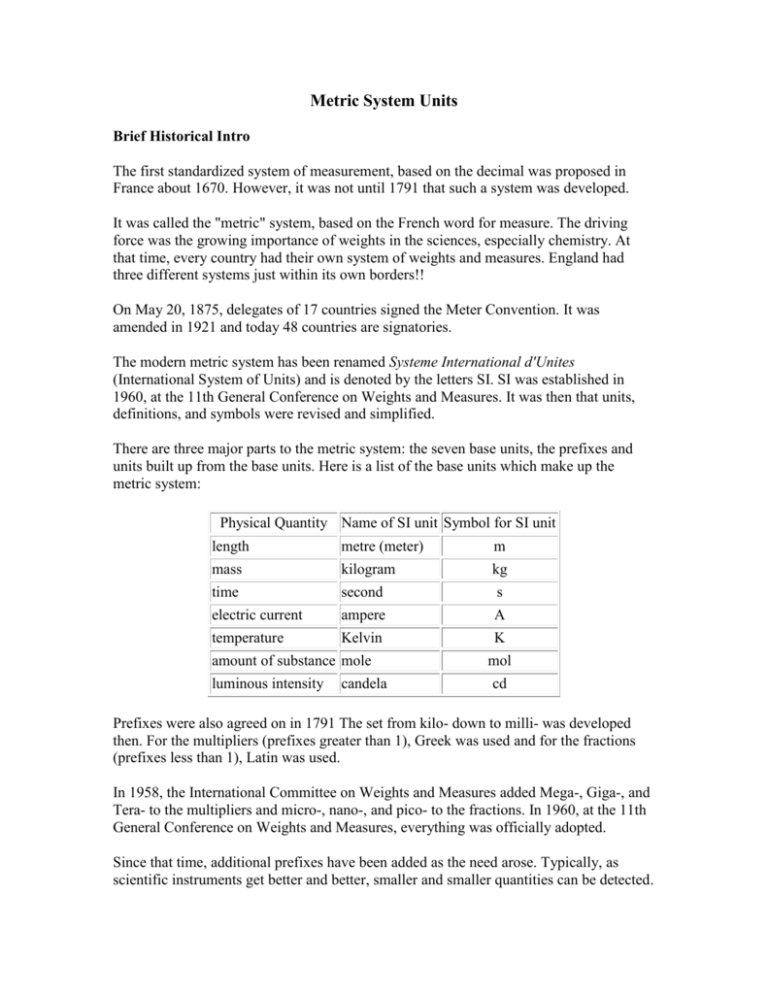
Metric System Units Brief Historical Intro The first standardized system of measurement, based on the decimal was proposed in France about 1670. However, it was not until 1791 that such a system was developed. It was called the "metric" system, based on the French word for measure. The driving force was the growing importance of weights in the sciences, especially chemistry. At that time, every country had their own system of weights and measures. England had three different systems just within its own borders!! On May 20, 1875, delegates of 17 countries signed the Meter Convention. It was amended in 1921 and today 48 countries are signatories. The modern metric system has been renamed Systeme International d'Unites (International System of Units) and is denoted by the letters SI. SI was established in 1960, at the 11th General Conference on Weights and Measures. It was then that units, definitions, and symbols were revised and simplified. There are three major parts to the metric system: the seven base units, the prefixes and units built up from the base units. Here is a list of the base units which make up the metric system: Physical Quantity Name of SI unit Symbol for SI unit length metre (meter) m mass kilogram kg time second s electric current ampere A temperature Kelvin K amount of substance mole luminous intensity candela mol cd Prefixes were also agreed on in 1791 The set from kilo- down to milli- was developed then. For the multipliers (prefixes greater than 1), Greek was used and for the fractions (prefixes less than 1), Latin was used. In 1958, the International Committee on Weights and Measures added Mega-, Giga-, and Tera- to the multipliers and micro-, nano-, and pico- to the fractions. In 1960, at the 11th General Conference on Weights and Measures, everything was officially adopted. Since that time, additional prefixes have been added as the need arose. Typically, as scientific instruments get better and better, smaller and smaller quantities can be detected. So, new fractional prefixes need to be added. When they are, new multipliers are added also, to keep the system symmetrical. Non-SI Units Commonly Used 1) Liter: symbol = L. The SI unit for volume is m3 (cubic meter). One dm3 (cubic decimeter) equals one L. A cubic decimeter is a cube 0.1 m on a side. 2) cubic centimeter: symbol = cm3. Often used for measuring the volume of solids, one cm3 equals one milliliter (mL). 3) Ångström: symbol = Å. One Å equals 10¯8 cm Definitions of Selected Units The Meter The meter has a most interesting history. The original definition was one ten-millionth of the distance from the North Pole to the Equator. From that, French scientists made a bar of 90% platinum and 10% iridium and put two marks on it to signify the meter distance. This particular alloy was used because it resisted expansions due to temperature very well and it could take a high polish, resulting in the ability to take a very fine line. This reduced the error due to the width of the lines. As science moved into the 1900s, it was becoming apparent that wavelength measurements were among the most accurate ones in all of science. In 1907, the red line of cadmium at 6438 Å was adopted as a new meter standard, however many continued to advocate the green line at 5460 Å in mercury's spectrum. By the way, using a particular wavelength of light as a standard for measurement was made as early as 1829. It took almost 80 years for the technology of measurement to become exact enough for use as an international standard. In 1960, the orange line at 6058 Å of krypton-86 was adopted. The wavelength was specified as : λvac = 6057.802106 Å so that one meter equaled: 1,650,763.73 Hz¯1 If you want to be really technical, this is the 2p10 to 5d5 transition (following the notation of Paschen). It can also be written: 2P10 to 5d5. So there! The definition was changed once again, in 1983, to the following: The meter is the length of path traveled by light in vacuum during a time interval of 1 / 299,792,458 of a second. By the way, this definition depends on the fact that the speed of light is defined (not measured) as exactly 299,792,458 meters per second. I think the meter's definition journey is over. The Kilogram An interesting fact about the kilogram is that it is the only SI base unit to incorporate a prefix. (By the way, teachers have been known to test that fact.) Why wasn't the gram used? I don't know for sure, but the gram is such a small amount of stuff that it would be easy to make a mistake in creating a standard. So I suspect they decided to deal with a larger amount of material. Here is the definition of the kilogram, adopted in 1901: it is equal to the mass of the international prototype of the kilogram. What that means is that there is a cylinder of 90% platnium/10% iridium in a vault in Paris, France and that lump of stuff weighs exactly one kilogram, by definition. I have heard, although I have no proof, that it has been removed for use only 4 times during the 1900s. The kilogram is the only unit still based on a single lump of stuff. All the other definitions can be reproduced with a high degree of accuracy in any laboratory with the proper equipment. Interestingly enough, the kilogram is losing weight and scientists are calling for the kilogram to be redefined in a more accurate way. Amount of Substance (The Mole) In 1860, at the first ever-international meeting of chemists, it was decided that hydrogen would serve as the standard for all atomic weights. It was defined as weighing exactly one atomic mass unit (amu). (The concept of mole had not yet been introduced into chemistry.) In 1906, oxygen was made the standard. There wasn't anything wrong with hydrogen, it was just that oxygen formed compounds with almost every element known and hydrogen did not. So oxygen was defined to weigh exactly 16 amu. However, in the 1940s (I think), the discovery of the O-17 and O-18 isotopes (both stable) changed matters, even though the two isotopes comprised only 0.24% of all oxygen atoms. Also, it was discovered that oxygen from different sources worldwide had slightly different mixes of the three isotopes. This was a BIG problem. In response, the physics community defined the amu as 1/16 the weight of the lightest oxygen isotope (which was O-16), while chemists do not come up with a new definition so fast. This meant that the atomic weights used by people in physics were different than those used in chemistry. This situation could not be tolerated. During the years 1958-1961 this issue was first debated by the worldwide chemistry and physics community and then voted upon. What emerged from the debate were two possible new standards: fluorine and carbon. The final vote showed carbon as the winner. Although fluorine had the advantage of only one natural isotope, carbon is much the safer element to handle. Also, while carbon has two natural, stable isotopes, the isotopic mix between carbon-12 and carbon-13 is well known and stable worldwide. These years were at the height of the Cold War between the USA and the Soviet Union. Despite this, the Soviet physics and chemistry communities were well represented in the debate and the voting. As a personal note, the Chemists were in elementary school during those years. I can still hear the air raid siren being tested every Friday at 10AM and I remember the drop-and-cover drills in case of nuclear attack (!). From 1971, the offical wording (unchanged to the present day) is as follows: The mole is the amount of substance of a system, which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon-12. When the mole is used, the elementary entities must be specified and may be atoms, molecules, ions, electrons, other particles, or specified groups of such particles. By the way, some books will rearrange the definition. For example: The amount of a substance of specified chemical formula, containing the same number of formula units (molecules, atoms, ions, electrons, etc.) as there are in 0.012 kilograms (exactly) of the pure nuclide carbon-12. This definition came from a reference book published in 1972 and is, of course, saying the same thing as the first definition. Metric Prefixes In order to properly convert from one metric unit to another, you must have the prefixes memorized. You will also need to determine which of two prefixes represents a bigger amount AND you will also need to determine the exponential "distance" between two prefixes. A metric prefix is a modifier on the root word and it tells us the unit of measure. For example, centigram means we are count in steps of one one-hundredth of a gram, μg means we count by millionths of a gram. A List of the Metric Prefixes Multiplier Prefix Symbol Numerical Exponential yotta Y 1,000,000,000,000,000,000,000,000 1024 zetta Z 1,000,000,000,000,000,000,000 1021 exa E 1,000,000,000,000,000,000 1018 peta P 1,000,000,000,000,000 1015 tera T 1,000,000,000,000 1012 giga G 1,000,000,000 109 mega M 1,000,000 106 kilo k 1,000 103 hecto h 100 102 deca da 10 101 no prefix means: 1 100 deci d 0.1 10¯1 centi c 0.01 10¯2 milli m 0.001 10¯3 micro μ 0.000001 10¯6 nano n 0.000000001 10¯9 pico p 0.000000000001 10¯12 femto f 0.000000000000001 10¯15 atto a 0.000000000000000001 10¯18 zepto z 0.000000000000000000001 10¯21 yocto y 0.000000000000000000000001 10¯24 Practice Problems There are three items - name, symbol, and size - that must be known. Problems could give any one and ask for one of both of the others. Here are only some possible problems (of many): I. Given either the name or the symbol of the prefix, give the other: 1) c 2) k 3) T 4) μ 5) d 6) milli 7) femto 8) giga 9) pico 10) hecto II. Given the prefix size, give its name: 11) 10¯15 12) 1,000 13) 109 14) 10¯2 15) 0.000001 Metric Prefix Answers I. Given either the name or the symbol of the prefix, give the other: 1) centi2) kilo3) tera4) micro5) deci6) m 7) f 8) G 9) p 10) h II. Given the prefix size, give its name: 11) femto12) kilo13) giga- 14) centi15) microThis next set of problems deserves some comment. The reason is that this particular skill isn't really mentioned by teachers. It seems that everybody just assumes kids pick it up somewhere. Probably because it's a real easy skill, but it is important. The skill I'm talking about is figuring out the absolute, exponential distance between two prefixes. For example, the absolute distance between milli and centi is 101. The distance between kilo and centi is 105. What you should do is compare the two exponents as if they were placed on a number line made of exponents and the compute the absolute distance between them. The key word is absolute. For example, someone might mentally do the distance between kilo and centi by comparing the exponents of 3 and negative 2 and getting one. So they reason the distance is 101. They would be wrong. Here is a number line with the two prefixes in problem sixteen marked: Compute the absolute, exponential distance between two given prefixes: 16) kilo and femto 17) milli and micro 18) micro and mega 19) centi and pico 20) nano and kilo 21) deci and tera 22) pico and micro 23) kilo and giga 24) femto and centi 25) milli and centi Metric Prefix Distance Answers Return to Metric Prefixes Return to Metric Table of Contents Compute the absolute, exponential distance between two given prefixes: 16) kilo and femto 103 and 10¯15 give a total absolute distance of 1018 17) milli and micro 10¯3 and 10¯6 give a total absolute distance of 103 18) micro and mega 10¯6 and 106 give a total absolute distance of 1012 19) centi and pico 10¯2 and 10¯12 give a total absolute distance of 1010 20) nano and kilo 10¯9 and 103 give a total absolute distance of 1012 21) deci and tera 10¯1 and 1012 give a total absolute distance of 1013 22) pico and micro 10¯12 and 10¯6 give a total absolute distance of 106 23) kilo and giga 103 and 109 give a total absolute distance of 106 24) femto and centi 10¯15 and 10¯2 give a total absolute distance of 1013 25) milli and centi 10¯3 and 10¯2 give a total absolute distance of 101 Some people are always looking for rules to memorize to help them. Notice that when the two prefixes are from the same side (two negatives or two positives), the answer is the absolute difference between the two. If the two prefixes cross over (as in one positive and one negative), then it is the absolute sum. OK, but what if the problem involves the base unit, as in "What is the absolute difference between nano and no prefix?" I guess the answer is that it doesn't matter because the absolute difference between 10¯9 and 100 is the same as the absolute sum. So there! Converting from One Metric Unit to Another Skills you need to do this include: 1) memorize the metric prefixes names and symbols 2) determine which of two prefixes represents a larger amount 3) determine the exponential "distance" between two prefixes 4) significant figure rules 5) scientific notation Here are two typical metric conversion problems: 1) Convert 2.50 μg to picograms. 2) Convert 0.080 cm to km. A slightly more complex one is: Convert the speed of light (3.00 x 108 m/sec) to km/year. We will concentrate on the first type in this tutorial. You can go to another tutorial which discusses the second type. The key skill in solving these problems is to construct a conversion factor. This conversion factor will make the old unit go away (micrograms and km in the top two examples) and create the new unit (pm and cm) in its place. Along with this change, there will be a change in the value of the number. Let's focus on the first example: Convert 2.50 μg to picograms STEP ONE: Write the value (and its unit) from the problem, then in order write: 1) a multiplication sign, 2) a fraction bar, 3) an equals sign, and 4) the unit in the answer. Put a gap between 3 and 4. All that looks like this: The fraction bar will have the conversion factor. There will be a number and a unit in the numerator and the denominator. STEP TWO: Write the unit from the problem in the denominator of the conversion factor, like this: STEP THREE: Write the unit expected in the answer in the numerator of the conversion factor. STEP FOUR: Examine the two prefixes in the conversion factor. In front of the LARGER one, put a one. There is a reason for this. I'll get to it in a second. STEP FIVE: Determine the absolute distance between the two prefixes in the conversion unit. Write it as a positive exponent in front of the other prefix. Now, multiply and put into proper scientific notation format. Don't forget to write the new unit. Sometimes, the exponential number is in the denominator. You must move it to the numerator and when you do so, remember to change the sign. Also, DO NOT move the unit with it. That unit has been cancelled and is no longer there. Here are all five steps for the second example, put into one image: Note that the old unit cancels, since it appears in the numerator and denominator of two parts of a multiplication problem. Why a one in front of the larger unit? I believe it is easier to visualize how many small parts make up one bigger part, like 1000 m make up one km. Going the other way, visualizing what part a larger unit is of one smaller unit, is possible, but requires more sophistication. For example, how many meters are in one nanometer? The answer is 0.000000001 or 10¯9. You may be able to handle the conversion and that is just fine. I'm just trying to make it simple. What I have been discussing above is sometimes called a "unitary rate." Unitary in this case simply means the number one. I have created a system above where the one can be in either the numerator or denominator of the conversion unit. You may have a teacher that forces the one to be only in the denominator. What that means is that you will have to decide if you are going from a large unit to a small unit (making the numerator exponent positive) or going from a small one to a large one (making the numerator unit negative). I think my way is better. Two Comments 1) If you do the conversion correctly, the numerical part and the unit will go in opposite directions. If the unit goes from smaller (mm) to larger (km), then the numerical part goes from larger to smaller. There will never be a correct case where number and unit both go larger or both go smaller. 2) A common mistake is to put the one in front of the SMALLER unit. This results in a wrong answer. Put the one in front of the LARGER unit. Practice Problems 1. 0.75 kg to milligrams 2. 1500 millimeters to km 3. 2390 g to kg 4. 0.52 km to meters 5. 65 kg to g Metric Conversion Problem Answers By the way, the answers to the two example problems are 2.50 x 10 6 pg and 8.0 x 10¯7 km. 1. 0.75 kg to milligrams Answer = 7.5 x 105 mg 2. 1500 millimeters to km Answer = 1.5 x 10¯3 km 3. 2390 g to kg Answer = 2.39 kg 4. 0.52 km to meters Answer = 520 m 5. 65 kg to g Answer = 6.5 x 104 g 6. 750 micrograms to g 7. 0.25 megameters to cm 8. 23.8 fg to kg 9. 2.77 kg to mg 10. 2.90 cm to terameters 11. 45.6 microliters to megaliters 12. 1.08 kg to μg 13. 9.57 x 10¯8 mm to nanometers 14. 2.00 L to mL 15. 35.28 mL to L Metric Conversion Problem Answers 6) 7.5 x 10¯4 g 7) 2.5 x 107 cm 8) 2.38 x 10¯17 km 9) 2.77 x 106 mg 10) 2.90 x 10¯14 Tm 11) 4.56 x 10¯11 ML 12) 1.08 x 109 μg 13) 9.57 x 10¯2 nm 14) 2.00 x 103 mL (2000 mL shows only one sig. fig.) 15) 0.03528 L Metric Conversion Special Note Convert 5000 cubic centimeters to mL and convert 3.5 L to cubic centimeters are of a type of conversion often seen. Cubic centimeters (cm3) is a non-SI unit, but it is in widespread use. So, here is the rule to memorize: one cm3 equals one mL. Prety easy rule to remember, but many students forget it and teachers do like to ask this type of conversion on tests. In the first example above, 5000 cm3 becomes 5000 mL and the second problem becomes "convert 3.5 L to mL." Then just replace mL with cm3 and the problem is complete (3500 cm3 is the answer, in case you were wondering.) Hopefully that was pretty easy. Just remember, it stands a decent chance of being on the test. Metric Square Units The unit which is most often squared or cubed is the length unit. In SI, the base length unit is the meter, m. A square unit, such as m2, describes area and a cubic unit, m3, describes volume. Brief Review With SI units (like meter, m), prefixes are added to indicate that a fraction (like centimeter, cm or 10¯2 m) or a multiple (like kilometer, km or 103 m) is being used. Suppose we wish to convert a value like 4.90 cm to the equivalent number of km. We need to do two things, (1) determine if we are converting to a bigger unit or smaller and (2) the absolute exponential distance between the two units. Smaller to bigger means a negative sign and the absolute exponential distance is 5. Therefore 4.90 cm = 4.90 x 10¯5 km Or, to use an even simpler example: 1.00 meter would equal 100. cm. The absolute exponential distance here is 2. Keep this problem in mind for the next few paragraphs. Converting from one square unit to another The big difference in this technique has to do with the exponential distance between two units. You must square the distance to get the right answer. Let's consider 1.00 m2, one square meter. How many square centimeters are there in this area? In order to solve this problem, you must ee that there are TWO sides to the square meter area and that EACH SIDE must be converted to cm. Consequently, we have 100.0 (102) cm on each side and when we multiply them together we get 104 cm2. Notice that the exponential distance is an exponent distance of 4 (that is, 104) where as in the meter to cm conversion, it was a distance of 2 (that is, 102) Let's do a longer distance. Consider 1.00 km2. Convert it to μm2 (square micrometers). Kilo is 103 and micro is 10¯6, so the total absolute exponential distance is 9 (which means 109). Since we are dealing with a square unit change, we must square 109, giving 1018 as an answer. We are going from one large unit (kilo-) to a smaller unit (micro-), so the sign on the answer will be positive: 1.00 km2 = 1.00 x 1018 μm2 Metric Cube Units Same explanation, except you have to cube the absolute exponential distance between to the two prefixes. I will do the same example problems as above, except as cubic problems. Remember that cubic problems deal with volume. Here are the problems: 1.00 m3 = how many cubic centimeters? 1.00 km3 = how many cubic micrometers? Before going into the solutions, try them out before seeing the answers. First problem: The absolute exponential distance between the base unit and centi- is 102 (sometimes a teacher may say the exponential distance is 2. Be aware that the 2 is an exponent, NOT that you will be multiplying someting by 2), so in a cubic situation, we must cube it to 106. The reason we do this is because there are three centimeter measurements, one for each of the three sides of the cube. Let me anticipate a problem: you read the above and say "Wait, the cube of 2 is 8, isn't it?" You are right because (2)3 = 2 x 2 x 2 = 8, but we are dealing with an exponential value of 2, not just the number 2. What gets cubed is 102, not 2. We would write (102)3 = 102 x 3 = 106. Hope that was clear. Now, to finish the problem: 1.00 m3 = 1.00 x 106 cm3 First problem: Between kilo- and micro- is an absolute distance of 109. Our technique calls for the exponential value to be cubed, so (109)3 = 1027. The direction of change is going from the larger prefix to the smaller prefix, so the sign of the exponent in the answer will be positive. Our answer is: 1.00 km3 = 1.00 x 1027 μm3 Practice Problems My examples above were going from the larger prefix to the smaller. That meant there were lots of the smaller prefix in one big prefix. The first problem in each set goes the opposite direction. Ask yourself: how many of a larger prefix are there in one small prefix? Answer = less than one, so the sign on the exponent will be negative. Having said that, watch out for a problem where there is an exponent already in the problem and the negative exponent change fails to overwhelm the exponent from the problem. In that case, there will be a positive exponent in the answer. Square problems 1) Convert 4.26 x 104 m2 to km2 2) Convert 3.20 x 1010 fm2 to cm2. 3) Convert the answer in number 2 to Mm2 Cubic problems 4) 4310 cubic centimeters. Convert to m3. 5) 86.3 cubic centimeters. What is volume in mm3? 6) 5.94 x 1010 mm3. Convert to dm3. Square Answers 1) The meter to km conversion = 103, therefore: m2 to km2 = 106 Since the conversion goes small unit (meter) to larger unit (km), we must make the sign on the exponent be negative, hence 10¯6. This means 4.26 x 104 m2 = 4.62 x 10¯2 km2 This creation of the negative can be seen in the following set-up which uses techniques explained previously: 2) The fm to cm conversion = 1013, therefore: fm2 to cm2 = 1026 Since the conversion goes small unit (femtometer) to larger unit (centimeter), we must make the sign on the exponent be negative, hence 10¯26. Therefore: 3.20 x 1010 fm2 x (1 cm2 / 1026 fm2) = 3.20 x 10¯16 cm2 3) no answer provided Cubic Answers 4) 5) 86.3 cm3 x (103 mm3 / 1 cm3) = 8.63 x 104 mm3 6) no answer provided Metric Unit Conversion Problems 1) How many palladium atoms would it take to encircle the Earth at its equator? (The atomic diameter of Pd is 140 pm; the equatorial diameter of the Earth is 40,075.02 km.) 2) In America, a car's gasoline efficiency is measured in miles/gallon. In Europe, it is measured in km/L. If your car's gas mileage is 40.0 mi/gal, how many liters of gasoline would you need to buy to complete a 142 km trip? Use the following conversions: 1 km = 0.6214 mi and 1 gal = 3.7854 L 3) Convert 2.1 Å to nanometers 4) Convert 0.0030 mm to nm 5) Convert 6.25 x 10¯4 s to ms Solutions to Metric Unit Conversion Problems 1) The absolute exponential distance between kilo- (103) and pico- (10¯12) is 1015. This problem can be done two ways. Method #1: Convert km to pm 40,075.02 km times (1015 pm / 1 km) = 4.007502 x 1019 pm 4.007502 x 1019 pm / (140 pm per atom) = 2.86 x 1017 atoms Method #2: Convert pm to km 140 pm times (1 km / 1015 pm) = 1.40 x 10¯13 km 40,075.02 km / (1.40 x 10¯13 km/atom) = 2.86 x 1017 atoms Comment: this amount of Pd is: 2.86 x 1017 / 6.022 x 1023 mol¯1 = 4.75 x 10¯7 mol and weighs: 4.75 x 10¯7 mol x 106.42 g mol¯1 = 5.06 x 10¯5 g 2) When I solved this problem, I did not clear the calculator at any point before rounding off the answer to three significant figures.