Chemistry Lab Manual: Safety & SI Units
advertisement

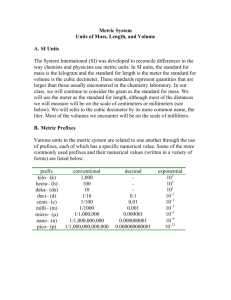
The Laboratory and SI Purpose: To check into the laboratory. To become familiar with the laboratory and laboratory manual. To learn laboratory safety rules and the necessity of practicing these rules in the laboratory. To develop skills in the use of le Système International d’Unités (SI Units). Background: All chemical principles, tools, and techniques are developed in the laboratory. The experience of observing a chemical phenomenon and then explaining its behavior is one that simply cannot be gained by reading a textbook or by listening to a lecturer. It is in the laboratory where chemistry comes alive, where chemical principles are learned and applied to the vast natural “chemistry laboratory” that we call our everyday environment. The objectives of a laboratory experience are to design and build apparatus, develop techniques, record and interpret data, and deduce rational theories so that the real world of science is better explained and understood. In the laboratory, you use common equipment and safe chemicals to perform experiments for collecting data. You record your experimental observations and try to interpret the data based upon sound chemical principles. A good scientist is a thinking scientist trying to account for the observed data and any contradictory data; cultivate self-reliance and confidence in your data, even if “it doesn’t look right” - this is how many breakthroughs in science have occurred. In the first few laboratory periods we will encounter some basic rules, equipment, and techniques and some situations where you will use them. These include laboratory safety rules, SI units, the Bunsen burner, and the balance. Procedure: A. Laboratory Check-In At the beginning of the laboratory period you will chose a lab station and lab partner. Tour the classroom and note the locations of all the glassware in cabinets. Whenever working in the lab make sure that all of the equipment is clean and in good condition. A good scientist is always neat and well-organized; keep your equipment clean and arranged in an orderly manner so that it is ready for use. Make sure that you restock the paper towels at your laboratory bench whenever necessary. Clean your laboratory area with wet paper towels and/or sponges at the conclusion of every experiment and whenever necessary during an experiment. 1 B. Laboratory Safety We will discuss laboratory safety and other basic laboratory procedures in class. Remember, however, that I cannot practice laboratory safety for you or for those who work with you-it is your responsibility to “play it safe.” Read and study the Laboratory Safety section. C. Le Système International d’Unités (SI Units) The SI, a modern version of the metric system, provides a logical and interconnected framework for all basic measurements. The SI, and some of its slight modifications, are used throughout the world by chemists and physicists as the international system for scientific measurements. For laboratory measurements, the SI base unit of mass is the kilogram (kg) (chemists are most familiar with the gram (g), where 10-3 g = 1 kg), the SI base unit for length is the meter (m), the SI base unit for volume is the cubic meter (m3) (chemists are most familiar with the liter (L), where 1 L = 10-3m3 = 1 dm3). Subdivisions and multiples of each base unit are related to these base units by a power of ten. The prefixes used to denote these subdivisions and multiples are shown in the following table: Table 1 Prefixes in SI Prefix Abbreviation Meaning (Power of Ten) Example using “grams” femto- f 10-15 fg = 10-15g pico- p 10-12 pg = 10-12g nano- n 10-9 ng = 10-9g micro- μ 10-6 μg = 10-6g milli- m 10-3 mg = 10-3g centi- c 10-2 cg = 10-2g deci- d 10-1 dg = 10-1g kilo- k 103 kg = 103g mega- M 106 Mg = 106g giga- G 109 Gg = 109g SI unit conversions are quite simple if the definitions for the prefixes are known and the factorlabel (dimensional analysis) method for problem solving is used. 2 The SI is compared to the English system in Table 2. Typical metric units are listed in brackets. Conversions between SI and the English system of weights and measures are quite valuable, especially to Americans because international science and trade communications are in SI or metric units. Table 2 Comparison of the SI (the Metric) and English Systems of Measurement Physical Quantity SI Unit Conversion Factor Length meter (m) 1 km = 0.6214 mi 1 m = 39.37 in 1 in = 0.0254 m = 2.540 cm Volume cubic meter (m3) 1 L = 10-3m3 = 1 dm3 = 103mL [liter (L)] 1 L = 1.057 qt 1 oz (fluid) = 29.57 mL Mass Pressure kilogram (kg) 1 lb = 453.6 g [gram (g)] 1 kg = 2.205 lb pascal (Pa) 1 Pa = 1 N/m2 [atmosphere (atm)] 1 atm = 101.325 kPa = 760 torr 1 atm = 14.70 lb/in2 (psi) Temperature Energy kelvin (K) K = 273 + oC [degrees Celsius (oC)] o joule (J) 1 cal = 4.184 J [calorie (cal)] 1 Btu = 1055 J C = 0.56 (oF - 32) 3