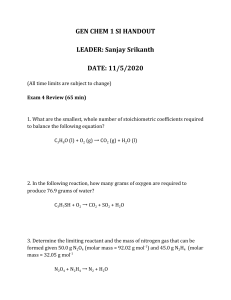
Applications of the Ideal Gas Law Density of a Gas
advertisement

Applications of the Ideal Gas Law Density of a Gas Example 1: Nitrogen gas is the most abundant gas in the atmosphere. What is the density of nitrogen gas, in g/L at 12.5oC and 126.63kPa? Solution: T = 285.5K P = 126.63kPa V = 1 (We can assume the volume is 1 since amount is not given) 1. Solve for moles: 2. Solve for mass 3. Calculate density PV = nRT Example 2: Oxygen is the second most abundant gas in the atmosphere. Calculate the density of oxygen at STP. We could make assumptions and follow the procedure above or we can use a little math help. Density = mass or Volume Proof: Molar Volume Molar Mass g/mol Molar volume L/mol g . = mol mol Solution: D=? MM = MV = Density = Molar Mass L