Chemical Reactions Study Guide
advertisement
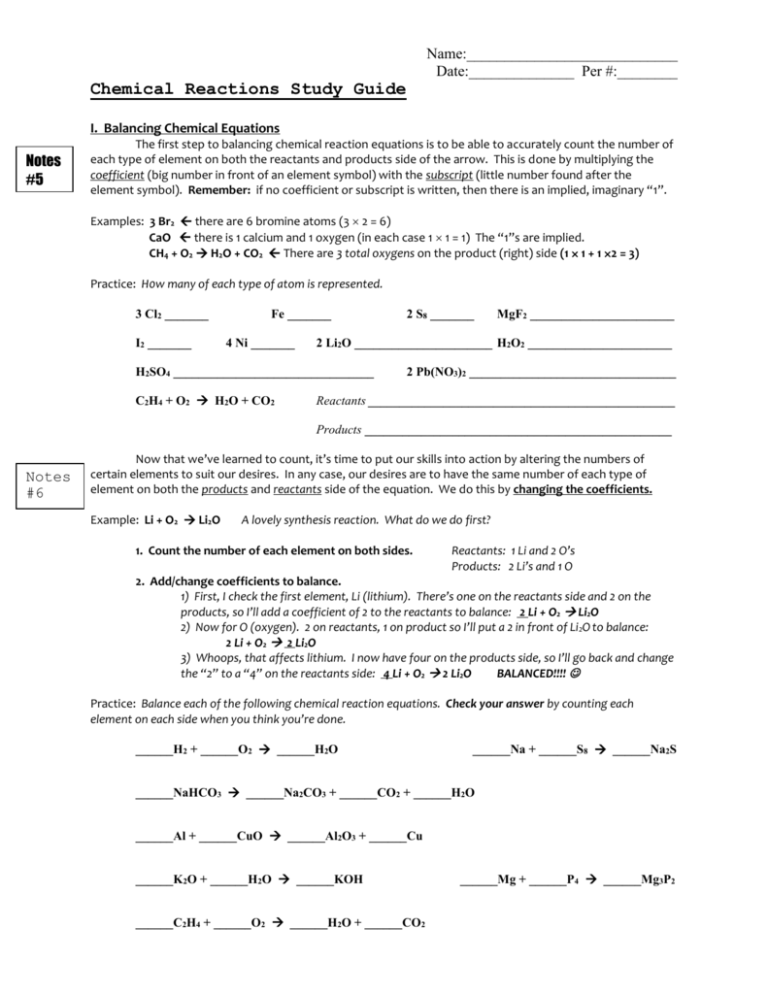
Name:____________________________ Date:______________ Per #:________ Chemical Reactions Study Guide I. Balancing Chemical Equations Notes #5 The first step to balancing chemical reaction equations is to be able to accurately count the number of each type of element on both the reactants and products side of the arrow. This is done by multiplying the coefficient (big number in front of an element symbol) with the subscript (little number found after the element symbol). Remember: if no coefficient or subscript is written, then there is an implied, imaginary “1”. Examples: 3 Br2 there are 6 bromine atoms (3 2 = 6) CaO there is 1 calcium and 1 oxygen (in each case 1 1 = 1) The “1”s are implied. CH4 + O2 H2O + CO2 There are 3 total oxygens on the product (right) side (1 1 + 1 2 = 3) Practice: H0w many of each type of atom is represented. 3 Cl2 _______ I2 _______ Fe _______ 4 Ni _______ MgF2 _______________________ 2 Li2O ______________________ H2O2 _______________________ H2SO4 ________________________________ C2H4 + O2 H2O + CO2 2 S8 _______ 2 Pb(NO3)2 _________________________________ Reactants _________________________________________________ Products _________________________________________________ Notes #6 Now that we’ve learned to count, it’s time to put our skills into action by altering the numbers of certain elements to suit our desires. In any case, our desires are to have the same number of each type of element on both the products and reactants side of the equation. We do this by changing the coefficients. Example: Li + O2 Li2O A lovely synthesis reaction. What do we do first? 1. Count the number of each element on both sides. Reactants: 1 Li and 2 O’s Products: 2 Li’s and 1 O 2. Add/change coefficients to balance. 1) First, I check the first element, Li (lithium). There’s one on the reactants side and 2 on the products, so I’ll add a coefficient of 2 to the reactants to balance: 2 Li + O2 Li2O 2) Now for O (oxygen). 2 on reactants, 1 on product so I’ll put a 2 in front of Li 2O to balance: 2 Li + O2 2 Li2O 3) Whoops, that affects lithium. I now have four on the products side, so I’ll go back and change the “2” to a “4” on the reactants side: 4 Li + O2 2 Li2O BALANCED!!!! Practice: Balance each of the following chemical reaction equations. Check your answer by counting each element on each side when you think you’re done. ______H2 + ______O2 ______H2O ______Na + ______S8 ______Na2S ______NaHCO3 ______Na2CO3 + ______CO2 + ______H2O ______Al + ______CuO ______Al2O3 + ______Cu ______K2O + ______H2O ______KOH ______C2H4 + ______O2 ______H2O + ______CO2 ______Mg + ______P4 ______Mg3P2 Name:____________________________ Date:______________ Per #:________ II. The Five Types of Chemical Reactions Notes #7, 8 This should be pretty simple. All you need to do is remember the names of the 5 types of rxns and the “Key to recognizing” each of them. As long as you understand what the symbols in a chemical reaction mean, it should be pretty simple. 1. Synthesis: Key = 2 reactants 1 product 2. Decomposition: Key = 1 reactant 2 products 3. Combustion: Key = products are only H2O and CO2 4. Single displacement: Key = there is a lonely (non-bonded) metal element on each side 5. Double displacement: Key = 2 ions switch places Examples: 2 K + F2 2 KF This is synthesis because two different reactants combine to make one product. 2 NaOH + CuSO4 Na2SO4 + Cu(OH)2 This is double displacement because the SO4 and OH ions switch places. Practice: Identify the type of reaction for each of the following reaction equations. 2 C3H6 + 9 O2 6 CO2 + 6 H2O Type? ____________________________________________ Zn + 2 AgF 2 Ag + ZnF2 Type? ____________________________________________ H 2O 2 H 2 + O 2 Type? ____________________________________________ Mg + Cl2 MgCl2 Type? ____________________________________________ NaCl + AgNO3 NaNO3 + AgCl Type? ____________________________________________ III. Acid and Base Chemistry Note s #9 There will be questions (multiple choice and otherwise) about the properties of acids and bases, i.e. their characteristics, pH ranges, the ions they produce when they dissolve in water, and the difference between strong and weak acids/bases. Study your notes for that stuff. The main free response stuff you’ll need to know is neutralization reactions. They are easy, there are 2 things you need to remember. 1) Neutralization reactions are a form of double displacement. 2) The products of neutralization reactions are always water and a salt. Example: HI + KOH H2O + KI Practice: Determine the products of each neutralization reaction. Then balance the equation. ______HCl + ______LiOH _____________ + _____________ ______HNO3 + ______NaOH _____________ + _____________ ______H2SO4 + ______KOH _____________ + __________________ ______HBr + ______Ba(OH)2 _____________ + _____________






