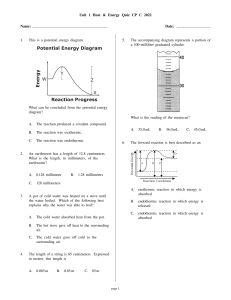
Measuring Changes of Heat and Temperature over time.
advertisement

Measuring Changes of Heat and Temperature over time. “It was the heat of the moment” Measuring heat • We cannot measure heat directly so we use changes in temperature as an indirect measure. • Heat Energy is also related to mass. • Heat Energy is also related to the type of substance or reaction taking place. Laws of Thermodynamics • 1 – The energy in the universe is constant • 2 – The universe is becoming more and more chaotic or less ordered. • 3 – As substances cool their entropy (or chaos) will approach zero. Specific Heat • The quantity of heat needed to raise the temp. of 1 gram of a substance 1 oC. • Units J / (g)oC • Energy is measured in joules. • Is related to an object’s mass. Common Objects SAMPLE SPECIFIC HEAT J/goC Water 4.1796 Gold 0.129 Iron 0.4498 Olive Oil 2.0 Heat Exchange (Change in Temperature) • Q = (cp) x (m) x (∆T) • Q = change in heat energy • cp = specific heat (relative to sample) • m = mass of sample • ∆T = Tf - Ti Exothermic v. Endothermic • Exothermic reactions release heat. • Endothermic reactions absorb heat. • (Q+) = heat is absorbed by the system • (Q-) = heat is released by the system Exercise • 300 g of Gold (c = 0.129 J/goC) is cooled from 295 K to 273 K. How much heat is removed from the system? Phase Changes and Heat • Temperature does not change during a phase change so a new equation is needed. • The amount of heat needed is dependent on two things: the amount of the substance and what the substance is. • So…… Cont’d • During a phase change… • Q = (m) x (heat of vaporization or fusion) • Hv and Hf hold true in both directions. • Liquid water to ice = -333.5 J/g • Ice to liquid water = 333.5 J/g Exercise • What is the heat of fusion for a 400g substance that requires 500 J to melt?