Fractional Distillation of Two Volatile Liquids
advertisement

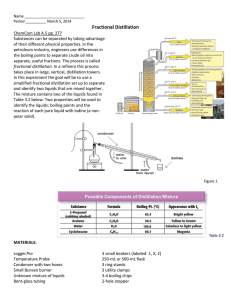
Fractional Distillation of Two Volatile Liquids Containing Non-volatile Impurities Pour 60 mL of mixture into a 100 mL round-bottomed flask. Add your magnetic stirring bar. Make note of the color and clarity of the mixture on your lab data sheet. Careful attention should be paid to the positioning of the thermometer. Have your instructor examine your apparatus before commencing heating. Place a 100 mL graduated cylinder so that the tip of the condenser is centered over the neck of the cylinder, as shown on the demonstration set-up. Place a 100 mL beaker directly under the suspended 100 mL graduated cylinder. Wrap aluminum foil around the Still head and still pot. Start the mixture stirring, heating initially at ”4”. Adjust heat so that the distillate comes over at About 1-2 drops/second. Collect the first fraction in your 100 mL graduated cylinder when the thermometer reaches a plateau. Record the temperature every 2 mL thereafter. When the temperature from one 2 mL reading to the next increases by more than 5 degrees, remove the 100 mL graduated cylinder, allowing subsequent drops to fall into the 100 mL beaker. Continue collecting liquid in the beaker and noting temperature readings at 2 mL intervals until a second plateau is reached. When the thermometer reaches the second plateau, collect the second fraction, in your 50 mL graduated cylinder, recording the temperature every 2 mL. Continue distillation for 2or 3 more 2 mL increments, recording each temperature. Note the volume of the first component recovered. Turn in this fraction in an appropriately labeled narrow mouth bottle. Record the volume of the second and third fraction collected, then, return them to the recovery bottle/flask in the front of the lab Plot a Temperature vs. Volume graph of your lab data on your lab report form. Che 301 Su’2008 Lab Data Sheet Title: Fractional Distillation Name:_____________________________________________________ Locker #:_____________ Date:______________________ Lab Data Liquid Used: _____________________________________________________________________ Appearance of the liquid ( circle the appropriate description(s) ) Colorless color cloudy clear other comments:_______________________________________________________________________ Volume of Liquid Used: mL. __ oC Temp. at which first drops enter the receiving vessel: Temperature/Volume Data: Fraction 1 Volume (mL) Temperature (oC) 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 10 12 14 16 18 20 22 24 26 28 30 10 12 14 16 18 20 22 24 26 28 30 Temperature/Volume Data: Fraction 2 Volume (mL) Temperature (oC) 2 4 6 8 Temperature/Volume Data: Fraction 3 Volume (mL) Temperature (oC) 2 4 6 8 Conclusions: (boiling range of first fraction) o C o (boiling range of third fraction) C Where would you change the receiver to collect fractions if you were trying to separate the components ? Calculate the mole fraction in a 1:1 (v/v) mixture using the data for the two components given below. Compound Boiling Range o C Molecular Weight Density gm/mL Volume mL Methanol 65 32.0 0.792 50 1-butanol 118 74.1 0.810 50