The aim of your research in this class is to clone
advertisement

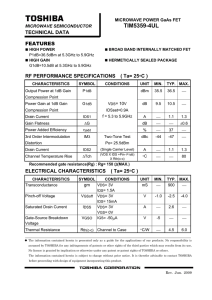
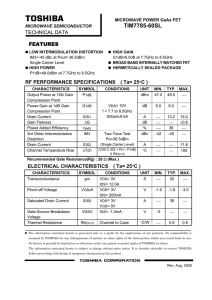
VDS of the FRI 533578592 *Put your name in the Header of EACH page Fall 12 *Use page numbers at bottom Final Research Report This report will take the form of a peer reviewed journal article, just like those that we have been reading in Journal Club. Format: the article should be displayed as TWO COLUMNS. There is an option to do this in WORD. You want your report to look like a journal paper. However, the TITLE and ABSTRACT should not be divided across two columns. See example image of the first page of a journal Page Length: Approximately 3,500 - 5,000 words. Estimate 8-10 pages Single Spaced (11 pt font) with figures and References. Don’t make any pictures bigger than about 1/3 rd of a page TITLE – give a title for your paper, succinct yet conveys what you are doing. Think like an advertising person here. AUTHORS – see a journal paper for example of an author and affiliation list, obviously put yourself there – but also might be a good idea to include the mentors and Dr. B unless you did all the work yourself! Also, if you collaborated with your classmates that worked on the same target – put their names. See the VDS poster in hall for how to spell names. ABSTRACT – this is a 150-200 word ‘blurb’ about what you did. It is a skeleton of your whole paper and should have the bare essentials necessary to allow a reader to skim it and determine if they care about reading your paper in full or not. Usually there is a sentence about the motivation then an overview statement or the project. Then brief methods and a few key pieces of data and results. Lastly, a short statement of conclusion. We practiced this in class (see Handout for tips) INTRODUCTION (a few paragraphs) Background on your target, motivation for this work (disease) Pull information from your proposal (Mid Semester Report onTarget). However, this should be now more refined taking out extraneous detail and including new relevant information. Be sure to include: Reaction Mechanism image (from BRENDA) for the natural substrate PyMol Image of protein zoomed out (showing overall structure and components – labels would be nice for the intro. Save the zoomed in version of the active site until you talk about virtual screening) Do not include random background pictures from the internet for your disease like you had in the proposal – unless it is a schematic type figure. A regular journal paper would not include these. MATERIALS & METHODS Use your revised versions of the M&M drafts that you turned in. But I would recommend changing the order to put Virtual last. Also you will need to generate the last M&M: Enzyme Inhibition Assays Cloning Start with an overview of the cloning process. Will need to explain non-standard methods (e.g. LIC cloning, GOLD) Include product names (Company Name, City, State) for non-common items When you list concentrations of reagents, you will almost always use the Final Concentrations of that reagent in the reaction volume (usually the final volume in the tube) Be sure to include Gene ID, CDS sequence, Codon Optimized sequence and All primers (Oligos, tail, sequencing) Expression and purification conditions OD at 600 nm using Red Tide Spec (Company, location) induced with IPTG (spell out) sonicator - type (may need to go look at it in the Biotech lab to get the name) Can post to Wiki if you want to share. pH adjustment prior to purification Centrifugal concentrators (?? MWCO – molecular weight cut off) mention size of your protein (does it get ‘caught’ by the MWCO filter Virtual Screening Include PyMol Image of active site zoomed in with the side chains for the active site well displayed . Don't need to mention Xming - it is just a program that allows you to see a Linux desktop from a Windows desktop Cluster specifics - # of blades, company, operating system, processors, memory, etc GOLD score components – see the VDS stream poster for more info (or the GOLD website) 1 VDS of the FRI 533578592 Fall 12 Enzyme assay & Inhibition assay If the substrate is different from the natural one that you talked about in the Intro, then show the different Reaction Mechanism (BRENDA). Reaction components: Substrates, buffer conditions, metals. Temperature, time, stop solution, method of measurement (absorbance), wavelength, what is the chromophore (i.e. what absorbs the light), spectrophotometer RESULTS & DISCUSSION This is where Figures and Tables will go. Matter-of-factly state and show what your results were. Then talk about your results and analyze them. Address each figure or table Then discuss their significance. Each Figure or Table should have a comprehensive caption explaining what is going on. Reader should not have to reference other parts of the paper in order to understand the Figure - Captions should include enough info to let the Figure stand alone • • Cloning gels (PCR, RE check – no plate images of colonies though!). Crop unnecessary ‘dead space’ from images Protein Characterization Gel (attests to purity and quantity) – don’t show Logger Pro image of OD600 growth (can show an Excel graph of OD600 growth) • Virtual Results – Final lists (from at least 2 libraries. These can be two separate tables or combined table) with Lipinski’s info (denote which ones don’t satisfy the rule) • Small (1.5 inches tall) 2-D images of 3 ligands • 3-D PyMol images zoomed in of active site and docked molecule for 3 different ligands – Show good shot of active site like in the original Research Report write up. – Analyzing your best ligand 1. Potential polar contacts (with hydrophilic amino acids – H bond donors/acceptors) 2. Hydrophobic interactions (with hydrophobic amino acids) • Enzyme Assay - should be in Excel (NOT a Logger Pro raw data graph with all pretty colors) – Your enzyme concentration should be in micromolar units (uM) or nanograms per microliter (ng/ul) units NOT in microliter volume. You will have to calculate this yourself using C1V1 = C2V2 • Inhibition Assay graphs (Absorbance at X nm wavelength [a.u.] on Y-axis and concentration of Inhibitor on x-axis) – Your inhibitor concentration should be in micromolar units (uM) NOT in microliter volume. What were the difficulties in the process? Were the results good/bad? Did the experiments/assays work? What were sources of error in your experiments that could make the results difficult to interpret or incorrect? CONCLUSIONS & FUTURE DIRECTIONS (1 paragraph – several sentences) Sum up what has been done (reiterating all that you have just said in one or two sentences) Then propose what your next steps will be (does not need to have specifics but must be detailed enough to show that you know the appropriate things to follow) REFERENCES • Cite at least 5 papers (one should be a review paper and one experimental) ACS (American Chemical Society) bibliography format. Use EndNote Web to do bibliography – Go to FORMAT > BIBLIOGRAPHY > ACS FORMAT > PLAIN TEXT FILE > PREVIEW and then copy and paste to your report at the end. APPENDIX – (optional) Can have your sequences of Oligos here. Can also put a gold.conf file here. TIPS: o Include citations [in brackets] in the body Put your name in the Header of EACH page of your paper Use page Numbers: p. 1, p. 2, p. 3 etc. o Don’t use ‘I’ Use third person Justify your margins to full width (i.e. block o Use paste tense – almost all of the time style paragraphs) o Don’t end a sentence in a preposition SUBMITTING Title your filename appropriately – using the standard format that we have been using. Convert your document to a PDF UTEID_Name _Date_ EnzymeName_VDS_FinalReport.pdf Upload to the DropitTome site. Passwerd: same as our GDocs http://www.dropitto.me/vdsclass 2