Compounds – Naming and Bonding REVIEW
advertisement
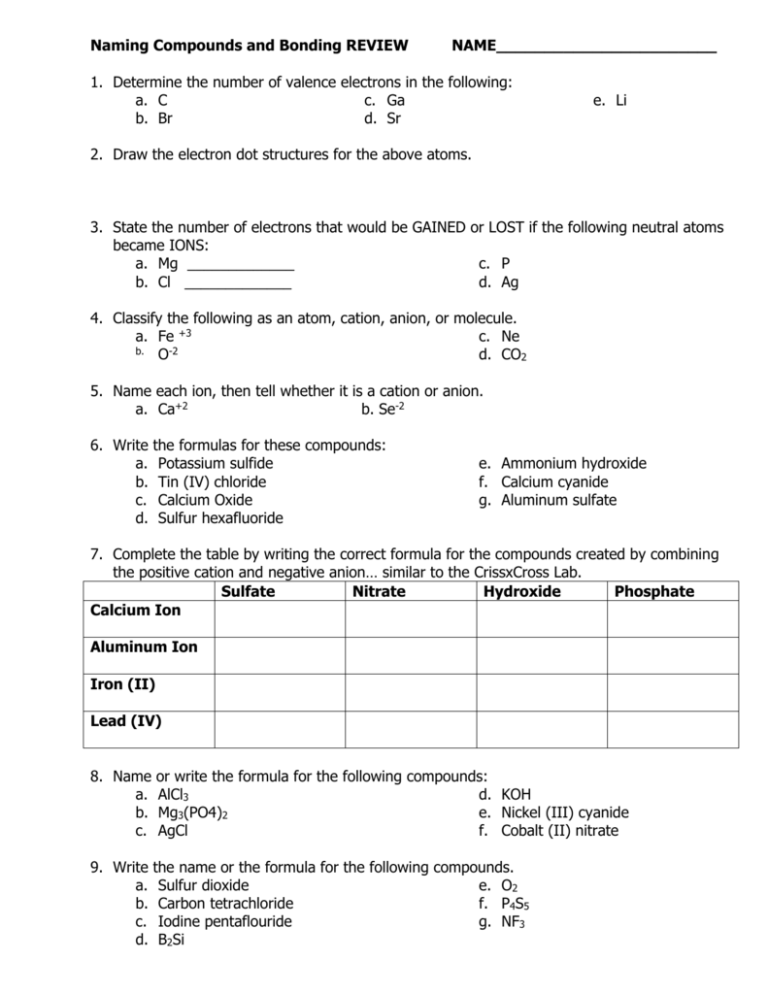
Naming Compounds and Bonding REVIEW NAME_______________________ 1. Determine the number of valence electrons in the following: a. C c. Ga b. Br d. Sr e. Li 2. Draw the electron dot structures for the above atoms. 3. State the number of electrons that would be GAINED or LOST if the following neutral atoms became IONS: a. Mg _____________ c. P b. Cl _____________ d. Ag 4. Classify the following as an atom, cation, anion, or molecule. a. Fe +3 c. Ne b. O-2 d. CO2 5. Name each ion, then tell whether it is a cation or anion. a. Ca+2 b. Se-2 6. Write a. b. c. d. the formulas for these compounds: Potassium sulfide Tin (IV) chloride Calcium Oxide Sulfur hexafluoride e. Ammonium hydroxide f. Calcium cyanide g. Aluminum sulfate 7. Complete the table by writing the correct formula for the compounds created by combining the positive cation and negative anion… similar to the CrissxCross Lab. Sulfate Nitrate Hydroxide Phosphate Calcium Ion Aluminum Ion Iron (II) Lead (IV) 8. Name a. b. c. or write the formula for the following compounds: AlCl3 d. KOH Mg3(PO4)2 e. Nickel (III) cyanide AgCl f. Cobalt (II) nitrate 9. Write a. b. c. d. the name or the formula for the following compounds. Sulfur dioxide e. O2 Carbon tetrachloride f. P4S5 Iodine pentaflouride g. NF3 B2Si 10. Draw the electron dot structures for the following compounds. a. CF4 c. F2 b. H2S d. AsCl3 11. Predict the shapes of the following molecules and tell if it’s polar/nonpolar: a. H2O b. HF c. CCl4 d. PH3 e. O2 12. Rank the following bonds from strongest to weakest: ionic, double covalent, triple covalent, single covalent, dispersion, hydrogen bond 13. Which are polar covalent and non-polar covalent: a. CCl4 b. H2 c. H2O d. NH3 14. What is a dipole? 15. Why do molecules form bonds? 16. What is the octet rule? If a molecule does not satisfy the octet rule for each atom in the molecule, what can you predict about its stability? 17. Name the differences between properties of ionic and covalent compounds. 18. What are metallic bonds? 19. Differences in electronegativity can be used to determine whether or not a compound is ionic or covalent in nature. Know how to interpret the results of different electronegativities (MAGIC NUMBER = 1.67) and determine whether a compound is ionic or covalent. ONLINE PRACTICE www.quia.com/quiz/258607.html http://www.iq.poquoson.org/hsscience/chemistry/chemicalbonding/hschemicalbondingquiz1_06 tlm.htm http://www.sciencegeek.net/Chemistry/taters/directory.shtml UNIT#3





