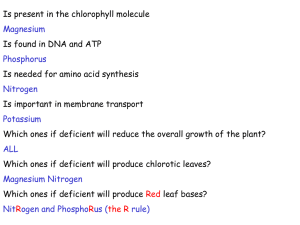
119. Bar A 2008 Calcium homeostasis, vitamin D metabolism and
advertisement

1 Preprint Review Calcium homeostasis and vitamin D metabolism and expression in strongly calcifying laying birds Arie Bar1 Institute of Animal Science, ARO, The Volcani Ctr., Bet Dagan, Israel Running head: Vitamin D in laying birds Published in: Comparative Biochemistry and Physiology, Part A: Molecular & Integrative Physiology, (2008) 151: 477-490. For the printed version please link to: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=186822 98 1 Retired; Institute of Animal Science, ARO, the Volcani Ctr., Bet Dagan 50250, Israel; E-mail: ariebar@agri.gov.il 2 ABSTRACT Egg laying and shell calcification impose severe extra demands on ionic calcium (Ca2+) homeostasis; especially in birds characterized by their long clutches (series of eggs laid sequentially before a “pause day”). These demands induce vitamin D metabolism and expression. The metabolism of vitamin D is also altered indirectly, by other processes associated with increased demands for calcium, such as growth, bone formation and egg production. A series of intestinal, renal or bone proteins are consequently expressed in the target organs via mechanisms involving a vitamin D receptor. Some of these proteins (carbonic anhydrase, calbindin and calcium-ATPase) are also found in the uterus (eggshell gland) or are believed to be involved in calcium transport in the intestine or kidney (calcium channels). The present review deals with vitamin D metabolism and the expression of the above-mentioned proteins in birds, with special attention to the strongly calcifying laying bird. Keywords: calbindin, calcium, carbonic anhydrase, eggshell gland (uterus), intestine, vitamin D (cholecalciferol) 3 Contents 1. Introduction ..........................................................................................................4 2. Calcium in avian reproduction .............................................................................4 2.1. Homeostasis: 4 2.2. Medullary bone 8 3. Vitamin D metabolism and its regulation in birds ...............................................8 3.1. Non-laying vertebrates 9 3.2. Laying birds 12 4. Vitamin D expression ..........................................................................................15 5. Vitamin D-dependent proteins .............................................................................16 5.1. Vitamin D receptor 16 5.2. Calbindins 17 5.3. Plasma membrane calcium-ATPase (Ca2+ATPase or PMCA) 23 5.4. Epithelial calcium channels (TRPVs) 24 5.5. Carbonic anhydrase 25 6. Vitamin D-controlled transcellular transport of Ca2+ .............................................................. 26 Concluding remarks ......................................................................................................................................... 27 Acknowledgments ............................................................................................................................................ 28 References .............................................................................................................................................................. 29 4 1. Intoduction Birds that lay long clutches (series of eggs laid sequentially before a “pause day”), among them the high-producing Gallus domesticus (domestic hen) and Coturnix coturnix japonica (Japanese quail) transfer about 10% of their total body calcium daily. This imposes severe demands on ionic calcium (Ca2+) homeostasis, and activates very efficient mechanisms for Ca2+ transfer from food to the eggshell. Two major calcium-regulating hormones are involved in these phenomena: the hormonally active vitamin D metabolite, 1,25-dihydroxy vitamin D3 (1,25(OH)2D3), and Parathyroid Hormone (PTH). Whereas PTH acts rapidly and directly – mostly on the kidney and bone – vitamin D acts more slowly, at the intestinal level also. Neither of these hormones has yet been found to affect eggshell gland (ESG; uterus) calcium transport directly, but many of the proteins believed to be vitamin D dependent are present and are modified in the ESG during egg laying by birds with long clutches. The present review focuses on the metabolism of the hormonal form of 1,25(OH)2D3 in laying birds; and on the expression of some out the many vitamin D-induced genes believed to be associated with Ca2+ transport in the intestine and ESG, rather than on the mechanisms of Ca2+ transport in the laying bird. These mechanisms will be discussed separately (Bar, in preparation). 2. Calcium in avian reproduction 2.1. Homeostasis: In order to maintain their extra-cellular Ca2+ concentration, land vertebrates implement homeostasis through a complex feedback mechanism that involves three major controlled systems, i.e., intestine, kidney and bone, and three major calcium-regulating hormones, i.e., PTH (reviewed in (Dacke 2000; Akerstrom et al. 2005; Potts 2005; Guerreiro et al. 2007; Potts et al. 2007)2, calcitonin, (reviewed in (Dacke 2000; Findlay et al. 2004; Huang et al. 2006) and 1,25-dihydroxycholecalciferol (1,25-dihydroxyvitamin D3; 1,25(OH)2D3) which is the hormonal form of cholecalciferol (vitamin D3; see 3). Changes (errors) in the plasma Ca2+ concentration are detected by Ca2+-sensing receptors (reviewed in (Miller 1992; Hurwitz 1996; Sasayama 1999; Ramasamy 2006). The controlled systems respond to detected errors in plasma Ca2+ concentration by adjusting the transport of Ca2+ into and out of the extracellular pool (Fig. 1). 2 In order to limit the review length, only few of the relevant reviews or original articles were cited. In most cases the most recent reviews and the earlier original papers were cited. In some cases were cited other (more relevant or more comprehensive) publications. 5 Fig. 1. Ca2+ homeostasis in the laying bird. Closed black arrows, Ca2+ flow; closed gray arrows, hormonal regulation; dotted black arrows, plasma Ca2+ regulation; dashed gray arrows, uncertain (controversial results) hormonal regulation (dCa/dt, the change in plasma Ca2+; PTH, parathyroid hormone; 1,25D3, 1,25(OH)2D3). Values given in parentheses are g calcium per day (Hurwitz et al. 1969). More recent studies indicated that the modern breeds/lines of laying domestic hens eat up to 5 g calcium per day and are able to deposit into the shell up to 2.7 g calcium per a single egg cycle (practically during the 2 nd half of the cycle). However, due to animal welfare restrictions/regulations, the recent publications are not distinguishing between fecal and urinary calcium excretion (as it requires a surgery procedure). 6 In birds, especially those with long clutches, egg laying and shell calcification introduces an additional Ca2+ pathway: its withdrawal through the uterus (egg shell gland, ESG). This imposes severe extra demands on Ca2+ homeostasis, since shell formation requires several times as much calcium as exists in the extra-cellular pool. These demands arise firstly through the initiation of gonadal activity during maturation prior to egg laying, to support new medullary bone (MB) formation and to saturate the estrogen-dependent plasma-calciumbinding proteins formed in the liver (reviewed in (Griffin 1992; Walzem 1996; Walzem et al. 1999)), and then from the enhanced requirements for shell Ca2+ during the egg-laying phase. The deposition of shell calcium appears not to be controlled by the three calciumregulating hormones, or, at least, to be controlled differently from Ca 2+ transport in the intestine, bone and kidney (Table 1; Bar et al. 1973b; Bar et al. 1975a; Bar et al. 1978b; Bar et al. 1984b; Bar et al. 1990b; Bar et al. 1992a; Bar et al. 1992c; Nys et al. 1989; Striem 1990). The intestinal lumen becomes almost completely empty of calcium 4 to 5 h after the end of the feeding stage (or when the light is turned off in birds with free access to food) (Hurwitz et al. 1966), whereas the intensive shell calcification in the commercial hen occurs between 9 and 22 h after ovulation, i.e., -1 to 12 h after lights-out, and consequently a major proportion of the shell Ca2+ is deposited while the intestine lacks adequate dietary calcium. Therefore, two mechanisms are enhanced: (a) enhancement of net absorption of Ca2+ during the dark period, especially early in that period when feed is still present in the gut (Hurwitz et al. 1965; Hurwitz et al. 1973; Bar et al. 1976b); and (b) the activation of an efficient process of bone resorption, mostly of the “medullary bone” (MB; details in the next paragraph.). Both mechanisms act during the period of shell calcification that occurs overnight. Restoration of the circadian bone loss of Ca2+ in birds that lay long sequential clutches occurs during the subsequent daylight period, when the intestinal absorption of Ca2+ is enabled by the renewal of calcium intake. 7 Table 1. Differential regulation of avian calbindin: Effects of selected physiological and nutritional alterations ______________________________________________________________________________ Cause/tissue Intestine Kidney ESG References1 ______________________________________________________________________________ VVitamin D derivatives in vitamin-D-deficient2 +++3 ++ =/+4 (Wasserman et al. 1966; Corradino et al. 1968; Taylor et al. 1972; Striem et al. 1991) 1-hydroxylated derivatives in vitamin-D-fed +++ = = (Bar et al. 1973c; Bar et al. 1975b; Bar et al. 1976b) Dietary Ca2+ restriction in vitamin-D-fed +++ =/- =/- (Wasserman et al. 1968; Bar et al. 1972a; Bar et al. 1973b; Bar et al. 1975b; Bar et al. 1978b) Dietary P restriction in vitamin-D-fed +++ +++ = (Morrissey et al. 1971; Bar et al. 1973c; Bar et al. 1975b; Bar et al. 1984a) = = ND5 (Bar et al. 1973c; Bar et al. 1975b) +++ +++ ND (Bar et al. 1973c; Bar et al. 1975b) High dietary Ca2+ in D-fed -- + ND (Bar et al. 1990a) Growth ++ = Maturation or gonadal hormones ++ = =/+ (Bar et al. 1972a; Bar et al. 1973b; Montecuccoli et al. 1977b; Bar et al. 1978b; Bar et al. 1979a; Navickis et al. 1979a; Navickis et al. 1979b) Laying +++ = +++ (Corradino et al. 1968; Wasserman et al. 1968; Bar et al. 1972a; Bar et al. 1973b; Bar et al. 1978b) Shell calcification (on protein synthesis) = ND = Shell calcification (on mRNA synthesis) = ND +++ Dietary Ca2+ restriction in 1-hydroxylated-D-fed Dietary P restriction in 1-hydroxylated-D-fed (Bar et al. 1981a) (Bar et al. 1975a) (Nys et al. 1989; Striem et al. 1991; Bar et al. 1992a; Nys et al. 1992a) ___________________________________________________________________ 1 The table tries to bring together most earlier published quantitative or semi-quantitative comparisons, as well as supporting evidence published later by other research teams. Many, but not all, other studies are mentioned in the text and in the References list. Few of the very early studies used the chelex assay, but were confirmed later with immunoassays or Western analysis. 2 Not laying. 3 The regulative response varied between non (=) to very strong (+++) or negative (-); ND, not detected; 4 Varied in accordance with laying rate. 5 Not determined. 8 2.2. Medullary bone: The unique MB is formed in the marrow cavities of the long bones of mature female birds (reviewed in (Dacke et al. 1993b; Gay 1996; Sugiyama et al. 2001; Beck et al. 2004; Whitehead 2004)). The MB accumulates rapidly during sexual maturation and the onset of egg production, and slow accumulation of its components continues throughout the laying period. The MB does not appear to have a structural function, but it serves as a labile source of Ca2+ for shell deposition. Such a labile source enables shell calcification in birds with long clutches or in birds that calcify eggs during the dark hours in the absence of an adequate intestinal supply of calcium (see 2.1). The formation and maintenance of MB require the combined effects of estrogen and androgen (Bloom et al. 1942); in addition, its mineralization requires the intestinal supply of Ca2+ and the active metabolite of vitamin D: 1,25(OH)2D3 (Kaetzel et al. 1984; Newbrey et al. 1992; Newman et al. 1999). During the 24- to 25.5-h cycle of egg formation in chicks and quails, MB is formed and resorbed by the osteoblasts and osteoclasts, respectively. The changes in the MB are manifested in its degree of calcification, rather than its volume (reviewed in (Dacke et al. 1993b)). Prior to the entry of an egg into the ESG, MB calcification/formation by osteoblasts is induced in response to estrogen secretion by mature follicles; its binding to specific receptors (reviewed in ( Sugiyama et al. 2001) occur mostly onthe osteoblasts plasma membrane (Armen et al. 2000). During shell calcification, the plasma Ca2+ concentration declines, which induces secretion of PTH (Dacke 1976; van de Velde et al. 1984; Singh et al. 1986) and its binding to specific receptors (Yasuoka et al. 1996) (reviewed in (Sugiyama et al. 2001)), and this, in turn, prompts the resorption by the osteoclasts. Whereas the roles of gonadal hormones and 1,25(OH)2D3 in MB formation, and of PTH in MB resorption are well established, the involvement of calcitonin, which is secreted by the ultimobranchial glands (Eliam et al. 1988; Dacke et al. 1993a; Sugiyama et al. 2001), and of Ca2+-sensing receptors (Zaidi et al. 1996) in MB remodeling are not yet certain. 3. Vitamin D metabolism and its regulation in birds The active metabolite of vitamin D3, i.e., 1,25(OH)2D3 was first known to affect the classical calcium-transporting organs such as the intestine, kidney and bone, as well as some non-classical organs and functions such as cell growth, immune response and apoptosis. Among the factors modulating Ca2+ absorption in birds, vitamin D appears to be the most important. Many other factors, such as dietary content of Ca2+ and/or phosphorus (P), 9 maturation and gonadal activity, egg laying and shell calcification, appear to modulate Ca2+ absorption, mainly through their effects on vitamin D metabolism and expression. 3.1. Non-Laying Vertebrates Metabolism: The metabolism and expression of vitamin D in non-laying land vertebrates have been extensively reviewed elsewhere (Bouillon et al. 1997; Henry 1997; Pike 1997; Haussler et al. 1998; Jones et al. 1998; Brown et al. 1999; Holick 2003; DeLuca 2004; Dusso et al. 2005; Norman 2006). Vitamin D metabolism in birds is basically similar to that in mammals, but was less extensively reviewed (Hurwitz 1992; Norman 1995; Soares et al. 1995; Wasserman 1997; Whitehead 1998; Edwards 2000). Briefly (Fig. 2): in birds (and in a few New World primates) the bioactivity of vitamin D3 is higher than that of ergocalciferol (vitamin D2), because the affinity of avian plasma vitamin D binding proteins (DBD) is markedly lower for vitamin D2 than for D3 derivatives, whereas the affinity of mammalian DBD (DeLuca et al. 1988) is similar for both. Vitamin D3, either from a dietary source or synthesized in the skin from 7-dehydroxycholesterol in response to ultraviolet irradiation and skin temperature is bioactivated, first in the liver and than in the kidney. This bioactivation (reviewed in (Omdahl et al. 2002; Ohyama et al. 2004; Prosser et al. 2004; Dusso et al. 2005)) involves the participation of three or more cytochrome P450 (CYP) isoforms. Vitamin D3 is first 25-hydroxylated in the liver microsomes and mitochondria to form 25-hydroxyvitamin D (25OHD3), which is the major circulating metabolite. The microsomal 25-hydroxylase in the liver (CYPR1) is capable of functioning at the physiological substrate concentration. Mitochondrial 25-hydroxylation appears to use another P450 isoform (out of at least six isoforms that exhibit vitamin D 25-hydroxylation activities). It appears to be species specific, and it also appears to use a non-specific pathway associated with vitamin D intoxication. Similarly to other vitamin D metabolites, 25OHD3 is mostly bound to DBD and appears to be slightly more active than vitamin D3 itself, apparently because of its better intestinal absorption (Bar et al. 1980). 10 Fig. 2. Vitamin D metabolism and regulation in the laying bird (EST, estrogens; GH, growth hormone; Pl-Ca2+, plasma Ca2+; Pl-P, plasma P-; PTH, parathyroid hormone). 11 Activation of 25OHD3 synthesis occurs in the kidney mitochondria, where it is further 1-hydroxylated by the 25-hydroxy-vitamin-D-1-hydroxylase (1-hydroxylase; CYP27B1), to form the hormonal form of vitamin D3, 1,25(OH)2D3. The kidney also expresses, a high activity of vitamin D-derived 24-hydroxylase (24-hydroxylase; CYP24A1), which is, most likely, responsible for inactivation of vitamin D derivatives. The renal vitamin D 24hydroxylase catalyzes side-chain cleavage and inactivation of 25OHD3 and 1,25(OH)2D3. Although the major site of activity of both 24- and 1-hydroxylases is the kidney, both are found in other tissues also, including the mammalian placenta, intestine, bone and skin. Regulation of 25OHD3 and 1,25(OH)2D3 formation and degradation: 25-hydroxylation in the liver is not strictly feedback regulated; it mainly reflects substrate availability. Therefore, the level of plasma 25OHD3 appears to be a good indicator of dietary vitamin D3 content or of vitamin D status. Renal 1-hydroxylation of 25OHD3 and the formation of 1,25(OH)2D3 is fine-regulated by plasma Ca2+ and PTH. Contradictory opinions have been expressed regarding the possible direct role(s) in 1,25(OH)2D3 formation of gonadal (Tanaka et al. 1976; Baksi et al. 1978; Bar et al. 1979a; Nys et al. 1984c; Sommerville et al. 1989; Striem 1990) or other hormones such as prolactin or growth hormone (Spanos et al. 1976) or dietary phosphorus (P) restriction (Henry et al. 1974; Ribovich et al. 1975; Montecuccoli et al. 1977b; Bar et al. 1981a), but their involvement is generally accepted. Dietary Ca2+ restrictions and rapid growth stimulate 1-hydroxylation, most likely through increased PTH secretion. In birds, but not in mammals, dietary P restriction does not induce renal 1-hydroxylation (Henry et al. 1974; Montecuccoli et al. 1977b), although the contents of the hormone in the plasma and target organs are increased (Edelstein et al. 1975; Hunziker et al. 1982; Rosenberg et al. 1986). This could be attributed either to an elevation in vitamin D receptor (VDR) (see 5.1) content or affinity, or possibly to retarded clearance of 1,25(OH)2D3 in P-restricted birds. Whereas there is some evidence to support the first explanation (Meyer et al. 1992), the clearance of 1,25(OH)2D3 in P-deficient birds has not yet been determined. Age and high levels of circulating 1,25(OH)2D3 moderate the responses of birds (Bar et al. 1981a) and mammals (Armbrecht et al. 1980a; Armbrecht et al. 1980b) to dietary minerals restriction or to growth. Acidosis in the chick is associated with a decreased 1-hydroxykase activity and/or plasma 1,25(OH)2D3 contents (Booth et al. 1977; Sauveur et al. 1977; Cunningham et al. 1987). Similar findings were obtained in mammals, including rats, cats and humans (Reddy et al. 1982; Cunningham et al. 1984; Ching et al. 1989). In humans, these effect are observed in 12 acute, but not in chronic acidosis (Cunningham et al. 1984). This reduction is attributed to an indirect effect of acidosis, manifested through plasma Ca2+ or PTH, but not plasma H+ (Bushinsky et al. 1985; Ro et al. 1990). The 24-hydroxylase is regulated in a reciprocal manner to 1-hydroxylase. The gene encoding vitamin D 24-hydroxylase contains at least two distinct vitamin D-responsive elements (VDREs) (see 5.1) that mediate the effects of 1,25(OH)2D3 via its receptor, on transcription. Although the present review deals mainly with 1,25(OH)2D3, the reader should be aware of the biological activities of other 25OH-metabolites, among which is 24,25(OH)2D3. This metabolite was found to be involved in: bone remodeling in mammals and birds (Henry et al. 1976; Ornoy et al. 1978), bone fracture healing (Lidor et al. 1990) and egg hatchability ((Henry et al. 1978) reviewed in (Norman et al. 2002)). However, these metabolites were not yet shown to have a significant role in Ca2+ transport in laying birds (Grunder et al. 1990). On the other hand, some authors (Hart et al. 1984; Harrison et al. 1986) doubted some of the above findings. 3.2. Laying Birds Basically, the metabolism of vitamin D and its regulation are similar in mammals and in both non-laying and laying birds. However, sexual maturation and the subsequent onset of egg-laying challenge the homeostasis of Ca2+ (see 2.1) and, consequently, modulate vitamin D metabolism and expression. 3.2.1. Regulation of 1,25(OH)2D3 formation: The renal formation of 1,25(OH)2D3 and its concentration in the plasma are markedly increased in the laying bird (Montecuccoli et al. 1977b; Baksi et al. 1978; Bar et al. 1981b). First, the combined elevation of plasma estrogens and androgens that occurs during maturation stimulates the renal formation of 1,25(OH)2D3, either directly (Tanaka et al. 1976; Castilo et al. 1979), or indirectly through the formation of MB and the binding of calcium to plasma proteins (Bar et al. 1979a). Later, the onset of laying further stimulates 1,25(OH)2D3 synthesis (Kenny 1976; Spanos et al. 1976; Montecuccoli et al. 1977b; Bar et al. 1981b; Nys et al. 1992a), most likely as a result of the increased need for Ca2+ for the shell (Bar et al. 1978a; Nys et al. 1986b; Nys et al. 1992c). The latter is associated with a decrease in plasma Ca2+ and an increase in plasma PTH (van de Velde et al. 1984; Nys et al. 1986a; Singh et al. 1986; Frost et al. 1990; Ieda et al. 2000; Sugiyama et al. 2001; Goto et al. 2002b). The changes in plasma Ca2+ and PTH are considered by some researchers to stimulate the renal-1-hydroxylation during the period of 13 shell calcification (see next paragraph). Further stimulation of 1,25(OH)2D3 synthesis is induced by dietary calcium restriction (even if due to brief food withdrawal or overnight starvation), laying rate (Bar et al, unpublished results), and/or during the “pause” day(s) at the end of a clutch (Montecuccoli et al. 1977b; Sedrani et al. 1977; Bar et al. 1984b). Age slows down renal-1-hydroxylation, but the mechanism of this effect is uncertain: some data suggest that age reduces the basal metabolism of vitamin D (Abe et al. 1982; Joyner et al. 1987) whereas other findings suggest that it mainly affects the adaptive mechanism, in accordance with the fluctuating demand for dietary Ca (Bar et al. 1987). Similar effects of age on the adaptation of vitamin D metabolism and expression were demonstrated also in mammals (Armbrecht et al. 1980b; Armbrecht et al. 1999). The effect of the egg cycle on the renal production and/or plasma concentration of 1,25(OH)2D3 has also aroused controversy. Some findings (Abe et al. 1979; Nys et al. 1986a; Nys et al. 1992c) indicated that renal production or plasma concentration increased 10 to 20 h post oviposition, in accordance with the diminished plasma Ca2+ and increased plasma PTH levels (van de Velde et al. 1984; Nys et al. 1986a; Singh et al. 1986; Frost et al. 1990; Ieda et al. 2000; Sugiyama et al. 2001; Goto et al. 2002b). However, other studies did not support these findings (Fig. 3; (Montecuccoli et al. 1977b; Sedrani et al. 1977; Bar et al. 1984b; Kaetzel et al. 1985; Soares et al. 1988)), or even suggested that 1,25(OH)2D3 production decreased during shell calcification (Kenny 1976). This controversy could arise from the duration of the ESG inactivity period during the “pause” day at the end of the clutch (Montecuccoli et al. 1977b; Sedrani et al. 1977). Nevertheless, even if 1,25(OH)2D3 synthesis is really stimulated during shell formation, this could induce either a non-genomic or a delayed rather than an acute genomic response, because the genomic response takes several hours to become evident (Lawson 1978). 14 Fig. 3. Vitamin D metabolism and calbindin expression during the egg cycle. , calbindin mRNA; , calbindin; , shell calcium; , calcium absorption capability; , normal cycle; , day of pause at the end of the clutch (no shell formation). Mean ± SE. Means designated by different letter are significantly different (P < 0.05) (with permission of: (Bar et al. 1976a; Montecuccoli et al. 1977b; Bar et al. 1992a; Bar et al. 1992d)). The comparison and interpretation of experimental results obtained from laying birds is quite difficult and may lead to mistaken or controversial interpretations because, whereas growth is a continuous process, egg formation and shell calcification are circadian in nature. This can account for some of the aforementioned disagreements in the findings concerning vitamin D metabolism and expression in the laying hen. Since the Ca2+ demand is the major factor regulating 1,25(OH)2D3 synthesis, any change in Ca2+ demand will affect 1,25(OH)2D3 synthesis. The loss of a massive proportion of the body Ca2+ in the laid eggshell induces the development of a physiological Ca2+ deficiency and acceleration of vitamin D metabolism. The overall loss of Ca2+ depends on the rate of egg production and on the Ca2+ content of each eggshell, the latter characteristic being independent of the laying rate. Both characteristics are easily affected by nutrition, environment and disease. 15 4. Vitamin D expression Vitamin D is expressed in all four tissues associated with calcium homeostasis in the laying bird. However, the present review will focus on the expression of the major transporting tissues, the intestine and the ESG, rather than on the kidney or bone (reviewed in (Hurwitz 1992; Norman et al. 1993; Gay 1996; Edwards 2000; Gay et al. 2000; Sugiyama et al. 2001; Whitehead 2004)). The action of 1,25(OH)2D3 on the Ca2+-transporting cells is initiated by its binding to vitamin D receptors (VDRs), which have been identified in the mammalian and the avian intestine and kidney (reviewed in (Pike 1997; Haussler et al. 1998; Holick 2003; DeLuca 2004; Young et al. 2004; Dusso et al. 2005; Norman 2006)), and in the avian ESG (Coty 1980; Takahashi et al. 1980), and the mammalian uterus and placenta, bone, parathyroid glands (PT), pancreas, cardiovascular system and in other tissues (reviewed in (Pike 1997; Walters 1997; Haussler et al. 1998; Dusso et al. 2005)). Free 1,25(OH)2D3 is transferred into the nucleus of the target cell, where it binds to the VDR. The binding of 1,25(OH)2D3 to VDR stimulates the synthesis of RNAs coding for several proteins. Among these proteins are the vitamin-dependent calcium-binding proteins, calbindin D28K (Wasserman et al. 1966; Bar et al. 1972a) or calbindin D9K (Bronner et al. 1975; Thomasset et al. 1981) in the avian or mammalian intestine, respectively; Ca2+ATPase (calcium pump, PMCA) (reviewed in (Stokes et al. 2003; Strehler et al. 2007)); and epithelial calcium channels (TRPVs) proteins (reviewed in: (Belkacemi et al. 2005; Hoenderop et al. 2005b; Niemeyer 2005)). These three groups of proteins appear to facilitate the transcellular mechanism of Ca2+ transport (reviewed in ((Bouillon et al. 2003; Bronner 2003; Wasserman 2004; Hoenderop et al. 2005b)). Many other genes/proteins appear also to be vitamin D dependent or related (vitamin D-induced/suppressed genes or those having VDREs). Among them are osteocalcin and osteopontin, collagen type I (reviewed in (Kream et al. 1997; Pike 1997; Haussler et al. 1998)), and carbonic anhydrase II (CA; (Drewe et al. 1988; Quelo et al. 1994). Among the latter, only CA (believed to be associated with Ca2+ transport in the ESG) will be addressed in this review. The formation of 1,25(OH)2D3 and its interaction with the nucleus are relatively slow. Whereas the response to the other key calcium-regulating hormone, PTH, is rapid (within minutes), the genomic effect of vitamin D or of its active metabolites requires 9 to 24, or 3 to 6 h, respectively (reviewed in (Lawson 1978)). 16 In addition to the slow genomic effects of the active metabolites of vitamin D, a rapid effect of 1,25(OH)2D3 on a vesicular transport mechanism that involves microtubules and microtubule-associated calbindin (transcaltachia) was reported by (Nemere et al. 1984), and it was extensively studied since then (reviewed in (Nemere et al. 1990; Norman et al. 2002; Larsson et al. 2003; Dusso et al. 2005)). Such a rapid response (within seconds to minutes) suggested that non-genomic responses were involved. Additional non-genomic responses were demonstrated for brush border membrane permeability, composition or fluidity (Bikle et al. 1980; Putkey et al. 1982; Rasmussen et al. 1982; Brasitus et al. 1986) (see also 5.1). The overall contribution of the rapid non-genomic responses to intestinal or ESG Ca2+ transport is not yet known. Among the proteins considered to be vitamin D dependent, only calbindin has been widely studied in birds; VDR and the other vitamin-dependent proteins have been less studied in birds in general, and much less studied in laying birds. Furthermore, most of the relevant studies on vitamin D derivatives in laying birds addressed their involvement in production traits rather the biological mechanisms associated with their activity. Vitamin D-dependent proteins 5. 5.1. Vitamin D receptor The binding of 1,25(OH)2D3 to VDR activates the formation of a heterodimer of VDR with a partner protein such as retinoid X receptor (RXR). Following the binding of 1,25(OH)2D3 to a VDR, the receptor is phosphorylated, and recruits an unaligned retinoic X receptor (RXR) to form a VDR-RXR heterodimer. The RXR-VDR-1,25(OH)2D3 complex recognizes and targets the gene through a high-affinity association with the VDREs in the gene-promoter region, and thereby stimulates the synthesis of RNAs coding for several proteins. VDREs were identified in the promoters of gene-coding calbindins, 24-hydroxylase, osteopontin, osteocalcin and others (reviewed in (DeLuca 2004)), and in PMCAs, (Glendenning et al. 2000), TRPVs (Weber et al. 2001) and CA (Quelo et al. 1994). However, some of the available data are not fully consistent with the hypothesis that all the isomers of PMCAs, TRPVs and calbindins are vitamin D dependent in all examined species or tissues. In the chicken, the intestinal VDR content is markedly increased during maturation, prior to the onset of egg production. At the onset of egg production it remains high or declines slightly, but still remains higher than in the immature female chick (Wu et al. 1994). Maturation or estradiol treatment induce the development of immature ESG tissues and markedly increase their VDR content (Striem et al. 1989; Striem 1990; Bar et al. 1992c; 17 Yoshimura et al. 1997). The ESG VDR content remains high also after the onset of production. This high content is maintained during a short (up to 9 d) chemically induced egg arrest (Bar et al. 1990b) or during a longer arrest caused by molt induction, although the mucosal tissue is regressed (Yoshimura et al. 1997). In the normal laying hen the ESG VDR concentration is 33 to 20% of the intestinal VDR content (Coty 1980; Bar et al. 1984b) and its gene expression oscillates during the diurnal egg cycle in close temporal association with eggshell calcification (Ieda et al. 1995). In addition to the nuclear VDR, membrane-bound receptor(s) (mVDR) linked to signal transduction appear to mediate the non-genomic rapid responses to 1,25(OH)2D3 and 24,25(OH)2D3 (reviewed in (Norman et al. 2002; Dusso et al. 2005; Norman 2006); see also 3.1 & 4). The nature of these receptors remains controversial. At least two distinct proteins have been identified: the thiol-dependent oxidoreductase ERp57 (1,25D3-MARRS), and annexin II. However, the involvement of the latter is not fully accepted. On the other hand, it was recently shown that in some cells the nuclear VDR is associated with caveolae that are present in the plasma membrane. Recently (Norman 2006) proposed a conformational ensemble model to describe how changes in ligand shapes of 125(OH)2D3 act through the VDR in different cellular locations, and can selectively mediate both genomic and nongenomic rapid responses of the VDR. 5.2. Calbindins Expression of this group of proteins, previously named CaBPs (Ca-binding proteins) differs (Table 1) among many cell types and species. High concentrations of calbindins are found in tissues characterized by their massive transport of Ca2+, such as intestine, kidney, placenta, uterus and ESG of birds (Wasserman et al. 1966; Taylor et al. 1967; Corradino et al. 1968; Bruns et al. 1978; Marche et al. 1978; Delorme et al. 1983). Lower concentrations of calbindins are found in other tissues associated with Ca2+ homeostasis and metabolism, such as bone, tooth cells and PT cells. These proteins are found also in tissues not directly associated with Ca2+ transport: the nervous tissues contain high concentrations, and the pancreas and testes contain low concentrations (reviewed in (Christakos et al. 1997; Thomasset 1997)). Whereas the mammalian intestine, uterus, placenta, and other tissues (including the kidney in a few species) contain mainly calbindin D9K, the avian and other lower species' tissues contain calbindin D28K. In the chick intestine and ESG, the protein is localized primarily in the absorptive cells and in the tubular gland cells, respectively (Lippiello et al. 1975; Jande et al. 1981; Wasserman et al. 1991). In avian species high 18 concentrations of calbindin was found in the intestine, kidney and the ESG (Wasserman et al. 1966; Taylor et al. 1967; Corradino et al. 1968). In the chicken and quail intestine, calbindin concentrations are higher in the proximal than in the distal segments (Taylor et al. 1967; Bar et al. 1976b). The calbindins are considered to facilitate the movement of Ca2+ inside the epithelial cells of the calcium-transporting organs. Although these proteins appear to be primarily associated with Ca2+ transport, they may also be involved in protecting the cells from high concentrations of Ca2+ or from cellular degradation via apoptosis (Christakos et al. 2003). In all three major Ca2+-transporting organs, the intestine, the kidney and the ESG of birds, concentrations of calbindin is closely correlated with Ca2+ transport (Taylor et al. 1969; Morrissey et al. 1971; Bar et al. 1975a; Bar et al. 1979a; Bar et al. 1984b). However, whereas the calbindin contents in the kidney and in the ESG are correlated with the mass of calcium transported (weight unit), the intestinal calbindin is correlated with calcium transport capability (% of absorption) (Fig. 43; (Bar et al. 1979b)). Fig. 4. Plasma calcium, intestinal and plasma calbindin, and intestinal calcium absorption as functions of calcium intake (from (Bar et al., 1979b); CaBP, calbindin; Ca, total calcium). 3 In the final proof figs 4 & 5 were switched over. 19 5.2.1. Calbindin and vitamin D: The calbindin gene promoter contains a VDRE (reviewed in (Christakos et al. 1997; DeLuca 2004))4. Three species of mRNAs are encoded in the avian intestine, kidney and ESG by the calbindn D28K gene. A major species (approximately 2.0 kb) and two minor ones (approximately 2.7 and 3.0 kb (Fig. 5c; lanes 1 & 2). All three species of mRNA are induced by 1,25(OH)2D3 in the avian intestine and kidney, but not in the ESG (Hunziker 1986; Mayel-Afshar et al. 1988; Bar et al. 1990a; Striem et al. 1991; Bar et al. 1992a). Intestinal calbindin synthesis reflects the changes in 1,25(OH)2D3 in the intestinal cell. These include the changes that result from exogenous supplementation of vitamin D or its derivatives (Wasserman et al. 1966; Bar et al. 1975b; Bar et al. 1976b; Bar et al. 1978b; Bar et al. 1990b; Striem et al. 1991; Bar et al. 1992a), dietary restriction of Ca2+ and/or P in non-laying birds (Wasserman et al. 1968; Morrissey et al. 1971; Bar et al. 1972b; Friedlander et al. 1977; Montecuccoli et al. 1977a), growth and age (Bar et al. 1981a; Bar et al. 2003). As in the intestine, the full modulation of renal calbindin mRNAs requires vitamin D metabolites, without which renal calbindin mRNAs levels are very low and are almost unaffected by dietary alterations. However, unlike intestinal calbindin, the renal calbindin, although significantly diminished in vitamin D-deficient chicks, did not completely disappear, and even retained part of its capability to be modulated in response to dietary alteration (Bar et al. 1975b; Bar et al. 1990a). Most of the available evidence does not support the idea that ESG calbindin D28k is vitamin D dependent: endogenous or exogenous 1,25(OH)2D3 (Bar et al. 1976b; Bar et al. 1988; Bar et al. 1990b) had no effect on ESG calbindin, whereas it did affect intestinal or renal calbindin; and ESG calbindin mRNAs was induced in the shell-forming, vitamin Ddeficient quail that was fed high dietary Ca2+ (Striem et al. 1991). Furthermore, dietary calcium that affected intestinal and renal calbindin, or P restrictions that affected intestinal and renal calbindin in accordance with its effects on vitamin D metabolism, did not affect synthesis of ESG calbindin or calbindin mRNAs (Bar et al. 1973b; Bar et al. 1978b; Bar et al. 1984a; Bar et al. 1984b; Bar et al. 1999; Ieda et al. 1999). Similarly, maturation prior to the onset of laying, associated with increased renal formation of 1,25(OH)2D3, induced the synthesis only of intestinal calbindin and calbindin mRNAs, but not of ESG or renal calbindin (Bar et al. 1972a; Bar et al. 1973b; Bar et al. 1978b; Bar et al. 1990b; Bar et al. 1992a; Bar et al. 1999; Goto et al. 2002b). 4 This sentence was removed from the final proof. 20 On the other hand, the presence of a considerable concentration of VDR in the ESG (Coty 1980; Bar et al. 1984), as well as the parallel fluctuations of ESG VDR (Ieda et al. 1995) and calbindin mRNAs during the egg cycle, are indicative of possible involvement of 1,25(OH)2D3 in ESG functionality (Bar et al. 1984b; Bar et al. 1990b). This idea is further supported by the single finding that injection of 1,25(OH)2D3 directly into the ESG lumen increased the ESG calbindin concentration (Ohira et al. 1998), and is also supported by the finding that 1,25(OH)2D3 has a slight effect on synthesis of ESG calbindin mRNA in vitro, or of calbindin in vivo in estrogen-treated immature female chicks (Corradino 1993; Corradino et al. 1993). Fig. 5. Visualization of calbindin RNAs from the duodenum and eggshell gland (ESG) by Northern blotting. (a) Effect of laying on duodenal calbindin RNAs: Lane 1, vitamin-D-deficient chick; lane 2, mature non-laying; lane 3, laying hen (from (Bar et al. 1992a)). (b) Effect of laying on ESG calbindin RNAs: Lane 1, laying hen duodenum; lane 2, ESG of mature non-laying; lane 3, ESG of laying hen during period of ESG inactivity; lane 4, ESG of laying hen during period of shell calcification (from (Bar et al. 1992a)). (c) Effect of shell calcification blocking of ESG calbindin RNAs: Lane 1, untreated laying hen; lane 2, laying hen treated with a single oral dose of the carbonic anhydrase inhibitor acetazolamide; lane 3, laying hen following forced immature oviposition (from (Bar et al. 1992b)). (d) Effect of shell quality: Lane 2, laying hen with shell-less eggs; lane 3, laying hen with normal eggs; lane 4, laying hen with broken or cracked eggs; above are shown the previous last eggs. Hens were sampled 17 h post oviposition (from (Bar et al. 1992b). Membranes were hybridized with oligonucleotide complementary to the mRNA sequence encoding amino acids 58-68 of chicken calbindin (with permission). Similar results were obtained using the quantitative “solution hybridization assay". 21 5.2.2 Reproduction and gonadal hormones: In the female bird intestinal calbindin mRNA (Striem et al. 1991; Bar et al. 1992a) and calbindin (Bar et al. 1972a; Montecuccoli et al. 1977b; Bar et al. 1978a; Bar et al. 1981b; Bar et al. 1990b; Bar et al. 1992a; Nys et al. 1992a; Wu et al. 1994; Bar et al. 1996) are moderately increased during sexual maturation. Onset of laying markedly increased calbindin mRNA (Nys et al. 1989; Bar et al. 1990b; Striem et al. 1991; Bar et al. 1992a; Nys et al. 1992a) and calbindin (Wasserman et al. 1968; Bar et al. 1973b; Bar et al. 1976a; Bar et al. 1978a; Bar et al. 1978b; Bar et al. 1992a) (Sugiyama et al. 2007) synthesis in the intestine and ESG. Molting (Yoshimura et al. 1997; Yosefi et al. 2003) and any other factor that arrests egg production (Bar et al. 1973b; Bar et al. 1973a; Bar et al. 1992a) markedly reduce the intestinal and ESG calbindin contents. A combined treatment of estradiol and testosterone may mimic the effect of maturation on intestinal calbindin (Bar et al. 1979a; Nys et al. 1984c; Striem et al. 1989; Striem 1990; Nys et al. 1992a) in vitamin D-fed female chicks, most likely indirectly, as a result of the increased demand for calcium (see 2.2) The role of gonadal hormones in the development of the oviduct is well established, and this raised an interest in their role in synthesis of ESG calbindin and other proteins. Whereas uterine calbindin D9K in mammals, but not calbindin D28K in mice (Gill et al. 1995), is noticeably induced by estrogens (reviewed in (Choi et al. 2005)), most researchers (Navickis et al. 1979b; Nys et al. 1989; Striem et al. 1989; Bar et al. 1990b; Striem 1990; Nys et al. 1992b; Corradino 1993; Corradino et al. 1993; Bar et al. 1996), but not all of them (Goto et al. 2002a), suggest that calbindin D28K synthesis in the avian ESG is only slightly induced by estrogens or sexual maturation. The observed maximal increment in ESG calbindin or its mRNAs, in response to maturation or estrogen treatment is one to two orders of magnitude smaller than that induced by shell calcification in the normal laying bird. These findings suggest that estrogens alone cannot account for the markedly elevated synthesis of calbindin mRNAs in the ESG of the shell-calcifying bird. On the other hand, the repetitive small effects of estrogens, together with the occurrence of an estrogen-responsive element on the 5'flanking region of the calbindin D28K promoter (Gill et al. 1995), support the idea that estrogens are involved in the mechanism of calbindin gene expression in the ESG. In addition, indirect effects may result from the influence of estrogens on oviduct development during maturation or from estradiol treatments (Striem 1990; Corradino et al. 1993; Berg et al. 2001). 22 Whereas testosterone does not affect ESG calbindin synthesis, progesterone and dexamethasone inhibit it (Bar et al. 1996; Goto et al. 2002a). Dexamethasone, but not progesterone, also inhibited synthesis of intestinal calbindin and its mRNA. Both prolonged the egg cycle but, whereas dexamethasone increased shell calcification, progesterone inhibited Ca2+ deposition onto the shell. This suggests that the effect of progesterone is specific and may be involved in the regulation of ESG calbindin synthesis. This hypothesis is supported by the finding that the plasma progesterone concentration oscillated during the diurnal egg cycle (Doi et al. 1980; Johnson et al. 1980; Nys et al. 1986a; Braw-Tal et al. 2004), in close temporal association with eggshell calcification, and peaked about 4 h before the next ovulation, at the time when shell calcification diminished and then stopped. The ESG remains in a refractory state prior to actual reproduction, and calbindin mRNAs (Nys et al. 1989; Striem et al. 1991; Bar et al. 1992a; Ieda et al. 1995) and calbindin (Bar et al. 1973b; Bar et al. 1978a; Bar et al. 1978b) begin to appear during calcification of the first eggshell. In the ESG, calbindin mRNAs oscillate during the diurnal egg cycle, between near zero and high concentrations, in close temporal association with eggshell calcification (Figs. 3, 5). Similarly to duodenal-calbindin, ESG-calbindin is lower in non-laying hens during molting (Yoshimura et al. 1997; Yosefi et al. 2003) or in hens that lay shell-less eggs (Nys et al. 1986b; Rabon et al. 1991; Bar et al. 1999) than in those that lay calcified eggs, and is lower in hens that form thin eggshells than in those that form thick ones (Bar et al. 1984b; Rabon et al. 1991; Bar et al. 1992b; Goto et al. 2002b). This suggests that Ca2+ transport in the ESG, similarly to that in the kidney, plays a major role in the synthesis of ESG calbindin mRNAs. The absence of a major effect of vitamin D or estrogens on ESG calbindin, the association of ESG calbindin mRNAs, but not of calbindin, with shell formation, and the relationship of ESG calbindin with the mass of Ca2+ transported, rather than with the transport capability (as in the intestine), suggests that Ca2+ transport in the ESG, similarly to that in the kidney, plays a major role in the synthesis of ESG calbindin mRNAs. In light of the above findings, it was hypothesized that the regulative mechanism for the synthesis of calbindin mRNAs in the ESG is a complex, multiple one, which involves, in addition to a major Ca2+-transport-related process, estrogen and other endocrine factors (Nys et al. 1989; Striem et al. 1991; Bar et al. 1992a; Nys et al. 1992b; Corradino 1993; Corradino et al. 1993; Bar et al. 1996; Bar et al. 1999). 23 5.3. Plasma Membrane Calcium-ATPase (Ca2+ATPase Or PMCA) The PMCAs use the energy stored in ATP to extrude Ca2+ out of the cell against the electrochemical gradient. This group of more than 30 isomers is encoded by at least four independent genes (PMCA1-4) (reviewed in: (Carafoli 1992; Howard et al. 1993; Bouillon et al. 2003; Belkacemi et al. 2005; Hoenderop et al. 2005b; Nijenhuis et al. 2005)) with two alternative, independent splicing sites. Whereas PMCA1 and PMCA4 are widespread, PMCA2 and PMCA3 are tissue specific. The PMCA1b is the predominant isomer expressed in the mammalian (reviewed in: (Howard et al. 1993; Nijenhuis et al. 2005)) and the chicken intestine (Melancon et al. 1970; Strittmatter 1972; Davis et al. 1987) and kidney (Qin et al. 1993a). In the intestine, kidney and placenta the PMCAs are located on the basolateral membrane of the epithelial cell toward which Ca2+ is transported (Borke et al. 1989a; Borke et al. 1989b; Borke et al. 1990). The specific involvement of the intestinal Ca2+ pump and PMCA genes in intestinal Ca2+ transport has been intensively studied, mostly in mammals (reviewed by (Hoenderop et al. 2002; Stokes et al. 2003; Hoenderop et al. 2005b)) and in non-laying birds (Davis et al. 1987; Wasserman et al. 1992; Cai et al. 1993; Pannabecker et al. 1995). The intestinal expression at the transcriptional level is modulated by vitamin D (reviewed in: (Zelinski et al. 1991; Wasserman et al. 1992), and also by a variety of factors that affect vitamin D metabolism (see 3.2), such as alterations in dietary Ca2+ and P concentrations (Wasserman et al. 1992; Cai et al. 1993; Armbrecht et al. 1994), age (Armbrecht et al. 1994), pregnancy and lactation in mammals (Zhu et al. 1998). Recently a VDRE sequence was identified in the PMCA1 gene (Glendenning et al. 2000). All these findings support the idea of vitamin-D-mediated modulation of the intestinal PMCA. On the other hand, a consistent stimulatory effect of 1,25(OH)2D3 on renal PMCA1 has not been established: 1,25(OH)2D3 did not stimulate, or even down-regulate renal PMCA1 (reviewed by (Hoenderop et al. 2005b)). There is little evidence to support the idea that estrogens also regulate PMCAs: In estrogen receptor knockout mice, renal, but not intestinal, PMCA was significantly reduced (Van Cromphaut et al. 2003). In ovariectomized mice, 17-estradiol supplementation induced intestinal PMCA1b mRNA expression, but to a lesser degree than TRPV(5 and 6), calbindin (Van-Abel et al. 2003), and PMCA activity in kidney membrane vesicles (Dick et al. 2003). Although less information is available with regard to the role of PMCAs in intestinal absorption of Ca2+ in laying birds, it is most likely similar to those in mammals and nonlaying birds. At least one study (Grunder et al. 1990) indicated that vitamin D 24 supplementation slightly increased the intestinal (jejunal) Ca2+ATPase activity in laying hens. Immunohistochemical and enzymatic evidence indicate that PMCAs are also present in the ESG of laying birds (Pike et al. 1975; Coty et al. 1982; Lundholm 1982; Grunder et al. 1990; Wasserman et al. 1991). Early evidence suggested that energy-dependent Ca2+ transport took place across the ESG wall (Schraer et al. 1970), where the PMCA is localized primarily in the apical-microvillar membrane of the tubular gland cells (Yamamoto et al. 1985; Wasserman et al. 1991; Arai et al. 1996) facing the ESG lumen, where shell calcification occurs. Unlike intestinal or renal PMCA, ESG PMCA was not modulated by 1,25-(OH)2D3 (Nys et al. 1984a) or was only slightly affected by it (Grunder et al. 1990). Estradiol treatment (Corradino et al. 1993; Qin et al. 1993b) stimulated ESG, but not intestinal (Qin et al. 1993a) ATPase activity or concentration, independently of the vitamin D status (Nys et al. 1984a; Corradino et al. 1993). Egg laying increased (Pike et al. 1975) and suppression of shell formation decreased the activity of PMCAs in the ESG. The association of PMCAs with the egg cycle remains controversial (Grunder 1983; Watanabe et al. 1989; Balnave et al. 1992). Thinning of the eggshell caused by p-p'-DDT and DDE in several species of birds was associated with a reduction in the ESG Ca2+ATPase activity. The accumulated data on the association between Ca2+ATPase and the loss of eggshell integrity in birds exposed to environmental pollution has raised special interest in the environmental aspects of this association (reviewed in (Lundholm 1997), see also 5.5). 5.4. Epithelial calcium channels (TRPVs) The TRP (Transient Receptor Potential) super-family of cations channel is widely distributed in animal tissues; they comprise more than 27 proteins involved in the transport of ions into cells in mammals. Their six subfamilies include the TRPVs (reviewed in: (Belkacemi et al. 2005; Hoenderop et al. 2005b; Niemeyer 2005; van de Graaf et al. 2007; Venkatachalam et al. 2007)). Two apical Ca2+ channels – TRPV5 and TRPV6, also known as ECaC1 and ECaC2 or CaT2 and CaT1, respectively – were identified in the 1,25(OH)2D3responding epithelia of the upper intestine, the distal nephron, bone, and the mammalian placenta and uterus (reviewed in (Peng et al. 1999; Belkacemi et al. 2005; Hoenderop et al. 2005b; van der Eerden et al. 2005; Lambers et al. 2006), all of which are characterized by massive Ca2+ transport. Studies with VDR knockout mice suggested that expression of the Ca2+ channel genes in the intestine and kidney are regulated by 1,25-(OH)2D3, and that the genes encoding them have a VDRE (reviewed in (Belkacemi et al. 2005; Brown et al. 2005; Hoenderop et al. 2005b)). Although most findings support the idea that TRPVs are 25 1,25(OH)2D3 dependent, another study (Weber et al. 2001) opposed this idea. Estrogens have a distinct, vitamin D-independent stimulating effect at the genomic level of TRPV6 (reviewed in (Hoenderop et al. 2005a; van Abel et al. 2005)); they also affect renal TRPV5 (Van Cromphaut et al. 2003). The effect of estrogen on TRPV6 may be mediated by an estrogen-responsive element (ERE) that has been identified on the promoter sequence of mouse TRPV6, but not on TRPV5 (Weber et al. 2001). However, the same study also suggested that the effects of estrogen on intestinal calcium absorption and renal calcium reabsorption are not mediated through altered TRPV expression, at least at the transcriptional level. Recent evidence is also indicative of estrogen dependency of TRPV6 expression in the mouse uterus (Lee et al. 2007). TRPV5 and TRPV6 are considered to facilitate the entry of Ca2+ into the epithelial cells of the calcium-transporting organs. The specific role of TRPVs in the transport of Ca2+ in the laying-hen intestine and ESG has not yet been studied. 5.5. Carbonic anhydrase The carbonic anhydrases (CA) are a group of zinc-containing enzymes that catalyze the reversible hydration of carbon dioxide. The CAs are involved in bone resorption and calcification, ion transport, acid-base metabolism, and the movement of respiratory gases. The CA family comprises three evolutionarily unrelated subfamilies without significant sequence homology. At least 16 different isomers were identified in mammalians (reviewed in (Esbaugh et al. 2006; Purkerson et al. 2007)) and several novel isozymes have also been identified in avians (Holmes 1977). The commonest of these isomers in avian tissues is CA-II (Holmes 1977). CAs were found in the avian kidney (Holmes 1977; Brown et al. 1982; Gabrielli et al. 1998), epiphysis (Dulce et al. 1960) – specifically in bone osteoclasts (Gay et al. 1974; Billecocq et al. 1990) – intestine (Nys et al. 1984b; Grunder et al. 1990; Gabriella et al. 1994) and ESG (Benesch et al. 1944; Bernstein et al. 1968) – specifically in the ESG epithelial cells (Arai et al. 1996). The CAs are present in other cells also, such as gastric parietal cells and salivary glands, where their main role is to generate H+ and HCO3- during acid-base regulation. The formation of HCO3- in the avian ESG appears to be of especial importance for the formation of CaCO3 in the shell, where it acts as the sole counter-ion for Ca2+. The dependency of CA on vitamin D is not yet clear: a VDRE region was identified in the CA-II gene (Quelo et al. 1994), and 1,25(OH)2D3 was found to regulate the transcription of CA-II in myelomonocytes (Lomri et al. 1992), and to induce their differentiation ((Billecocq et al. 1990; Lomri et al. 1992). Vitamin D3 deficiency caused a reversible 26 reduction in CA activity in the ESG of the laying hen (Grunder et al. 1990), but CA activity was found to be unrelated to plasma level, or to exogenous supplementation of 1,25(OH)2D3 (Nys et al. 1986b; Grunder et al. 1990). CA inhibitors, such as acetazolamide, rapidly (within 10 to 12 h post administration) blocked shell calcification (Bernstein et al. 1968; Pearson et al. 1977; Eastin et al. 1978; Bar et al. 1992a; Bar et al. 1999) in spite of the availability of dietary calcium and Vitamin D3, and reduced ESG and intestinal calbindin (Bar et al. 1992a; Bar et al. 1999) and ESG Ca2+ATPase (Lundholm 1990). Of interest is the fact that acetazolamide in vitro reduced CA activity, but not Ca2+ transport (Ehrenspeck et al. 1971). This is indicative of an indirect role of CA in Ca2+ transport. Thinning of the eggshell caused by p-p'-DDT and DDE in quail was associated with a reduction in the ESG CA activity (Bitman et al. 1970). 6. Vitamin D-controlled transcellular transport of Ca2+ In birds and mammals the overall Ca2+ transport reflects a sum of saturated and unsaturated processes. Whereas the saturated process corresponds to active transcellular transport, the unsaturated process consists of diffusion through a paracellular route (reviewed in (Wasserman 1997; Bouillon et al. 2003; Bronner 2003; Wasserman 2004; Hoenderop et al. 2005b; van de Graaf et al. 2007)). Briefly, the transcellular transport consists of three major steps: entry of Ca2+ through the brush border, diffusion or movement to the basal membrane, and extrusion through the basal membrane. The first step proceeds down the chemical gradient of Ca2+ that results from its low cellular concentration (10-7 M) and high plasma and intestinal lumen concentrations (>10-3 M). This step appears to be facilitated by TRPV6 (see 5.4) and to a lesser extent by TRPV5. The second step appears to be facilitated by intracellular calbindins (Feher 1984; Feher et al. 1992; Koster et al. 1995). There is some evidence for calbindin-mediated cytosolic-free diffusion of Ca2+ and of vesicular transport (Nemere 1992).. The energydependent third step proceeds up the chemical gradient of Ca2+ that results from the low cellular Ca2+ concentration and the higher plasma Ca2+ concentration (1.25 to 1.50 mM). This step is facilitated by PMCA (see 5.3) and, to a lesser extent, by a sodium-calcium (Na+/Ca2+) exchanger (reviewed in (Hoenderop et al. 2005b; Lytton 2007)). Components of all three steps of transcellular transport are vitamin D-dependent (see 5), because TRPV6 and TRPV5, calbindins and PMCA all comprise a VDRE and/or are unaffected in the tissues of VDRknockout animals, and/or are stimulated in normal animals by 1,25(OH)2D3 or by factors that 27 affect its formation, such as dietary Ca2+, age, pregnancy and lactation in mammals, or egg laying in birds. Doubt is cast on the significance of transcellular transport in the intestine of the laying hen by the high Ca2+content of the intestinal lumen, because the electrochemical potential difference (ECPD) gradients in laying birds fed commercial high-calcium diets is sufficient to ensure a massive paracellular passive transport (Hurwitz et al. 1968) through the tight junctions. The possible involvement of 1,25(OH)2D3 in paracellular transport through the tight junctions is still a matter of controversy (McCormick 2002; Wasserman 2004). This issue is to be addressed and discussed with regards to the laying birds in another manuscript (Bar 2009). The findings of recent studies indicate that it is possible to control the tight junction (TJ) proteins (that connect between epithelial cells and form a biological barrier), permeability and paracellular transport (reviewed in (Schneeberger et al. 2004; Van Itallie et al. 2006)). It appears that 1,25(OH)2D3 and VDR, as well as cellular Ca2+, are among the factors responsible for preserving the integrity of TJ complexes, up-regulating TJ proteins, and modulating TJ permeability and paracellular Ca2+ transport (Tang et al. 2003; Kutuzova et al. 2004; Fujita et al. 2008; Kong et al. 2008). These findings support the hypothesis that 1,25(OH)2D3, also, may regulate paracellular Ca2+ transport. In the ESG, although TRPVs were not yet identified, the presence of the other two components (PMCA and calbindin) of transcellular transport Ca2+, the localization of PMCA (see 5.3), the presence of TRPVs in mammals uterus and placenta (reviewed in (Lee et al. 2007)) and the apparently uphill transport of Ca2+ that occurs there, support the idea that Ca2+ is transported via the transcellular pathway. However, the lack of an effect of vitamin D on the ESG transport of Ca2+, and the obvious and critical involvement of CA in this procedure, suggest that the mechanisms there could not be easily explained solely in terms of simple trancellular and/or paracellular mechanisms of Ca2+ transport. This issue is to be addressed and discussed extensively in another manuscript (Bar 2009). 6. Concluding remarks Shell calcification of birds with long clutches imposes severe demands on Ca2+ homeostasis, which is maintained by the highly efficient Ca2+ transport mechanisms that operate in the intestine, bones and the ESG. As an external Ca2+ supply is not guaranteed throughout shell-calcification period, these birds extract a significant portion of the shell Ca2+ 28 from the medullary bone reserves. Therefore, within a period of one egg cycle, the intestine not only supplies the major proportion of the shell Ca2+; it also replenishes most of the Ca2+ removed previously from the bone. Vitamin D is the major factor regulating intestinal transport. Its metabolism in laying birds is similar to that in non-laying higher vertebrates, although the birds do not use vitamin D2 metabolites efficiently, and their metabolism is somewhat differently regulated by plasma P. The birds' Ca2+ needs, as manifested through their plasma and PTH Ca2+ contents, appear to form the most important regulatory factor of vitamin D metabolism. Therefore, in these birds any factor capable of modifying Ca2+ demands, such as the clutch length, the shell mass and the egg cycle, consequently modulates vitamin D metabolism. This leads to some disagreements regarding interpretation of the findings on vitamin D metabolism obtained in different studies. Vitamin D expression in the intestine (the bone is not reviewed here) appears also to be similar to that known in non-laying higher vertebrates. Although the TRPVs have not yet been identified in birds, it most likely that the transcellular Ca2+ transport is also similar. However, because of the high calcium intakes of these birds, the paracellular pathway appears to be more important, especially during the period of shell calcification. The recent findings on the effect of calcium and vitamin D metabolites on TJ proteins and permeability offer new insights into Ca2+ transport in laying birds. The importance of vitamin D for ESG Ca2+ transport is less well understood and more complicated (ESG transport will be discussed in a later review; in preparation): although many of the vitamin D-dependent proteins are present in the ESG, in most cases, they do not respond to vitamin D. Some of them, such as the vitamin D-dependent calbindin, are differently regulated, and not necessarily in a vitamin D-dependent manner. On the other hand, ESG CA, which is most likely also a vitamin D-dependent protein, may play a determinant role in this tissue. Acknowledgments Contribution from the Institute of Animal Science, Agricultural Research Organization, the Volcani Center, Bet Dagan, Israel: No. 516-08. Encouragement and comments by Prof. S. Yahav, ARO, are acknowledged with appreciation. 29 References Abe, E., Horikawa, H., Masumura, T., Sugahara, M., Kubota, M., Suda, T., 1982. Disorders of cholecalciferol metabolism in old egg-laying hens. J. Nutr. 112, 436-446. Abe, E., Tanabe, R., Suda, T., Yoshiki, S., 1979. Circadian rhythm of 1 , 25-dihydroxyvitamin D3 production in egg-laying hens. Biochem. Biophys. Res. Commun. 88, 500-507. Akerstrom, G., Hellman, P., Hessman, O., Segersten, U., Westin, G., 2005. Parathyroid glands in calcium regulation and human disease. Ann. N. Y. Acad. Sci. 1040, 53-58. Arai, T., Sugiyama, T., Kusuhara, S., 1996. Immunohistochemical studies of Ca-2+-ATPase and carbonic anhydrase II in the shell gland of egg-laying hens. Jap. Poult. Sci. 33, 371-376. Armbrecht, H.J., Boltz, M.A., Wongsurawat, N., 1994. Expression of plasma membrane calcium pump mRNA in rat intestine: effect of age and 1,25-dihydroxyvitamin D. Biochim. Biophys. Acta 12, 110-114. Armbrecht, H.J., Hodam, T.L., Boltz, M.A., Kumar, V.B., 1999. Capacity of a low calcium diet to induce the renal vitamin D 1-hydroxylase is decreased in adult rats. Biochem. Biophys. Res. Commun. 255, 731734. Armbrecht, H.J., Zenser, T.V., Davis, B.B., 1980a. Effect of age on the conversion of 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 by kidney of rat. J. Clin. Invest. 66, 1118-1123. Armbrecht, H.J., Zenser, T.V., Gross, C.J., Davis, B.B., 1980b. Adaptation to dietary calcium and phosphorus restriction changes with age in the rat. Am. J. Physiol. 239, E322-E327. Armen, T.A., Gay, C.V., 2000. Simultaneous detection and functional response of testosterone and estradiol receptors in osteoblast plasma membranes. J. Cell. Biochem. 79, 620-627. Baksi, S.N., Kenny, A.D., 1978. Vitamin D metabolism in Japanese quail: gonadal hormones and dietary calcium effects. Am. J. Physiol. 234, E622-628. Balnave, D., El Khatib, N.U., Zhang, D., 1992. Calcium and carbonate supply in the shell gland of hens laying eggs with strong and weak shells and during and after a rest from lay. Poult. Sci. 71, 2035-2040. Bar, A., 2009. Calcium transport in strongly calcifying laying birds: Mechanisms and regulation. Comp Biochem Physiol A Mol Integr Physiol. (in press-on line) Bar, A., Cohen, A., Edelstein, S., Shemesh, M., Montecuccoli, G., Hurwitz, S., 1978a. Involvement of cholecalciferol metabolism in birds in the adaptation of calcium absorption to the needs during reproduction. Comp. Biochem. Physiol. B 59, 245-249. Bar, A., Cohen, A., Eisner, U., Risenfeld, G., Hurwitz, S., 1978b. Differential response of calcium transport systems in laying hens to exogenous and endogenous changes in vitamin D status. J. Nutr. 108, 13221328. Bar, A., Dubrov, D., Eisner, U., Hurwitz, S., 1976a. Calcium-binding protein and calcium absorption in the laying quail (Coturnix coturnix japonica). Poult. Sci. 55, 622-628. Bar, A., Eisner, U., Montecuccoli, G., Hurwitz, S., 1976b. Regulation of intestinal calcium absorption in the laying quail: independent of kidney vitamin D hydroxylation. J. Nutr. 106, 1336-1342. Bar, A., Hurwitz, S., 1972a. Relationship of duodenal calcium-binding protein to calcium absorption in the laying fowl. Comp. Biochem. Physiol. B 41, 735-744. Bar, A., Hurwitz, S., 1973a. Calcium restriction and intestinal calcium-binding protein in the laying fowl. Comp. Biochem. Physiol. A 45, 571-577. Bar, A., Hurwitz, S., 1973b. Uterine calcium-binding protein in the laying fowl. Comp. Biochem. Physiol. A 45, 579-586. Bar, A., Hurwitz, S., 1975a. Intestinal and uterine calcium-binding protein in laying hens during different stages of egg formation. Poult. Sci. 54, 1325-1327. Bar, A., Hurwitz, S., 1979a. The interaction between dietary calcium and gonadal hormones in their effect on plasma calcium, bone, 25-hydroxycholecalciferol-1-hydroxylase, and duodenal calcium-binding protein, measured by a radioimmunoassay in chicks. Endocrinology 104, 1455-1460. 30 Bar, A., Hurwitz, S., 1981a. Relationships between cholecalciferol metabolism and growth in chicks as modified by age, breed and diet. J. Nutr. 111, 399-404. Bar, A., Hurwitz, S., 1984a. Egg shell quality, medullary bone ash, intestinal calcium and phosphorus absorption, and calcium-binding protein in phosphorus-deficient hens. Poult. Sci. 63, 1975-1979. Bar, A., Hurwitz, S., 1987. Vitamin D metabolism and calbindin (calcium-binding protein) in aged laying hens. J. Nutr. 117, 1775-1779. Bar, A., Hurwitz, S., Cohen, I., 1972b. Relationship between duodenal calcium-binding protein, parathyroid activity and various parameters of mineral metabolism in the rachitic and vitamin D-treated chick. Comp. Biochem. Physiol. A 43, 519-526. Bar, A., Hurwitz, S., Edelstein, S., 1975b. Response of renal calcium-binding protein. Independence of kidney vitamin D hydroxylation. Biochim. Biophys. Acta 411, 106-112. Bar, A., Maoz, A., Hurwitz, S., 1979b. Relationship of intestinal and plasma calcium-binding protein to intestinal calcium absorption. FEBS Lett. 102, 79-81. Bar, A., Norman, A.W., 1981b. Studies on the mode of action of calciferol. XXXIV. Relationship of the distribution of 25-hydroxyvitamin D3 metabolites to gonadal activity and egg shell formation in the quail. Endocrinology 109, 950-955. Bar, A., Rosenberg, J., Hurwitz, S., 1984b. The lack of relationships between vitamin D 3 metabolites and calcium-binding protein in the eggshell gland of laying birds. Comp. Biochem. Physiol. B 78, 75-79. Bar, A., Shani, M., Fullmer, C.S., Brindak, M.E., Striem, S., 1990a. Modulation of chick intestinal and renal calbindin gene expression by dietary vitamin D 3, 1,25-dihydroxyvitamin D3, calcium and phosphorus. Mol. Cell. Endocrinol. 72, 23-31. Bar, A., Sharvit, M., Noff, D., Edelstein, S., Hurwitz, S., 1980. Absorption and excretion of cholecalciferol and of 25-hydroxycholecalciferol and metabolites in birds. J. Nutr. 110, 1930-1934. Bar, A., Shinder, D., Yosefi, S., Wax, E., Plavnik, I., 2003. Metabolism and requirements for calcium and phosphorus in the fast-growing chicken as affected by age. Br. J. Nutr. 89, 51-60. Bar, A., Striem, S., Mayel Afshar, S., Lawson, D.E., 1990b. Differential regulation of calbindin-D28K mRNA in the intestine and eggshell gland of the laying hen. J. Mol. Endocrinol. 4, 93-99. Bar, A., Striem, S., Rosenberg, J., Hurwitz, S., 1988. Egg shell quality and cholecalciferol metabolism in aged laying hens. J. Nutr. 118, 1018-1023. Bar, A., Striem, S., Vax, E., Talpaz, H., Hurwitz, S., 1992a. Regulation of calbindin mRNA and calbindin turnover in intestine and shell gland of the chicken. Am. J. Physiol. 262, R800-R805. Bar, A., Vax, E., Hunziker, W., Halevy, O., Striem, S., 1996. The role of gonadal hormones in gene expression of calbindin (M(r)28,000) in the laying hen. Gen. Comp. Endocrinol. 103, 115-122. Bar, A., Vax, E., Striem, S., 1992b. Relationships between calbindin (Mr 28,000) and calcium transport by the eggshell gland. Comp. Biochem. Physiol. B 101, 845-848. Bar, A., Vax, E., Striem, S., 1999. Relationships among age, eggshell thickness and vitamin D metabolism and its expression in the laying hen. Comp. Biochem. Physiol. A 123, 147-154. Bar, A., Wasserman, R.H., 1973c. Control of calcium absorption and intestinal calcium-binding protein synthesis. Biochem. Biophys. Res. Commun. 54, 191-196. Bar, A., Wasserman, R.H., Fullmer, C.S., 1992c. Study of the action of vitamin D in egg shell gland of the laying hen. In: United States – Israel Binational Agricultural Research and Developmen, Report # IS1278-87, Bet Dagan, Israel, pp. 34-38 Bar, A., Wasserman, R.H., Fullmer, C.S., 1992d. Study of the action of vitamin D in egg shell gland of the laying hen. In: United States – Israel Binational Agricultural Research and Developmen, Report # IS1278-87, Bet Dagan, Israel, pp. 91-112 Beck, M.M., Hansen, K.K., 2004. Role of estrogen in avian osteoporosis. Poult. Sci. 83, 200-206. Belkacemi, L., Bedard, I., Simoneau, L., Lafond, J., 2005. Calcium channels, transporters and exchangers in placenta: a review. Cell Calcium 37, 1-8. 31 Benesch, R., Barron, N.S., Mawson, C.A., 1944. Carbonic anhydrase, sulphonamides and shell formation in the domestic fowl. Nature 153, 138-139. Berg, C., Holm, L., Brandt, I., Brunstrom, B., 2001. Anatomical and histological changes in the oviducts of Japanese quail, Coturnix japonica, after embryonic exposure to ethynyloestradiol. Reproduction 121, 155-165. Bernstein, R.S., Nevalainen, T., Schraer, R., Schraer, H., 1968. Intracellular distribution and role of carbonic anhydrase in the avian shell gland mucosa. Biochem. Biophys. Acta 159, 367-376. Bikle, D.D., Whitney, J., Munson, S., 1984. The relationship of membrane fluidity to calcium flux in chick intestinal brush border membranes. Endocrinology 114, 260-267. Billecocq, A., Emanuel, J.R., Levenson, R., Baron, R., 1990. 1 alpha 25-dihydroxyvitamin D3 regulates the expression of carbonic anhydrase II in nonerythroid avian bone marrow cells. Proc. Natl. Acad. Sci. U S A 87, 6470-6474. Bitman, J., Cecil, H.C., Fries, G.F., 1970. DDT-induced inhibition of avian shell gland carbonic anhydrase: a mechanism for thin eggshells. Science 168, 594-596. Bloom, M.A., McLean, F.C., Bloom, W., 1942. Calcification and ossification: the formation of medullary bone in male and castrate pigeons under the influence of sex hormones. Anat. Rec. 83, 99-120. Booth, B.E., Tsai, H.C., Morris, R.C., Jr., 1977. Metabolic acidosis in the vitamin D-deficient chick. Metabolism 26, 1099-1105. Borke, J.L., Caride, A., Verma, A.K., Kelley, L.K., Smith, C.H., Penniston, J.T., Kumar, R., 1989a. Calcium pump epitopes in placental trophoblast basal plasma membranes. Am. J. Physiol. 257, C341-C346. Borke, J.L., Caride, A., Verma, A.K., Penniston, J.T., Kumar, R., 1989b. Plasma membrane calcium pump and 28-kDa calcium binding protein in cells of rat kidney distal tubules. Am. J. Physiol. 257, F842-849. Borke, J.L., Caride, A., Verma, A.K., Penniston, J.T., Kumar, R., 1990. Cellular and segmental distribution of Ca2(+)-pump epitopes in rat intestine. Pflugers Arch. 417, 120-122. Bouillon, R., Carmeliet, G., Boonen, S., 1997. Ageing and calcium metabolism. Bailliere Clin Endocrinol. Met. 11, 341-365. Bouillon, R., Van Cromphaut, S., Carmeliet, G., 2003. Intestinal calcium absorption: Molecular vitamin D mediated mechanisms. J. Cell. Biochem. 88, 332-339. Brasitus, T.A., Dudeja, P.K., Eby, B., Lau, K., 1986. Correction by 1-25-dihydroxycholecalciferol of the abnormal fluidity and lipid composition of enterocyte brush border membranes in vitamin D-deprived rats. J. Biol. Chem. 261, 16404-16409. Braw-Tal, R., Yossefi, S., Pen, S., Shinder, D., Bar, A., 2004. Hormonal changes associated with ageing and induced moulting of domestic hens. Br. Poult. Sci. 45, 815-822. Bronner, F., 2003. Mechanisms of intestinal calcium absorption. J. Cell. Biochem. 88, 387-393. Bronner, F., Freund, T., 1975. Intestinal CaBP: A new quantitative index of vitamin D deficiency in the rat. Am. J. Physiol. 229, 689-694. Brown, A.J., Dusso, A., Slatopolsky, E., 1999. Vitamin D. Am. J. Physiol. 46, F157-F175. Brown, A.J., Krits, I., Armbrecht, H.J., 2005. Effect of age, vitamin D, and calcium on the regulation of rat intestinal epithelial calcium channels. Arch. Biochem. Biophys. 437, 515-518. Brown, D., Roth, J., Kumpulainen, T., Orci, L., 1982. Ultrastructural immunocytochemical localization of carbonic anhydrase. Presence in intercalated cells of the rat collecting tubule. Histochemistry 75, 209213. Bruns, M.E., Fausto, A., Avioli, L.V., 1978. Placental calcium binding protein in rats. Apparent identity with vitamin D-dependent calcium binding protein from rat intestine. J. Biol. Chem. 253, 3186-3190. Bushinsky, D.A., Riera, G.S., Favus, M.J., Coe, F.L., 1985. Response of serum 1,25(OH)2D3 to variation of ionized calcium during chronic acidosis. Am. J. Physiol. 249, F361-365. Cai, Q., Chandler, J.S., Wasserman, R.H., Kumar, R., Penniston, J.T., 1993. Vitamin D and adaptation to dietary calcium and phosphate deficiencies increase intestinal plasma membrane calcium pump gene expression. Proc. Natl. Acad. Sci. U S A 90, 1345-1349. 32 Carafoli, E., 1992. The Ca2+ pump of the plasma membrane. J. Biol. Chem. 267, 2115-2118. Castilo, L., Tanaka, Y., Wineland, M.J., Jowsey, J.O., DeLuca, H.F., 1979. Production of 1,25dihydroxyvitamin D3 and formation of medullary bone in the egg-laying hen. Endocrinology 104, 15981601. Ching, S.V., Fettman, M.J., Hamar, D.W., Nagode, L.A., Smith, K.R., 1989. The effect of chronic dietary acidification using ammonium chloride on acid-base and mineral metabolism in the adult cat. J. Nutr. 119, 902-915. Choi, K.C., Leung, P.C., Jeung, E.B., 2005. Biology and physiology of Calbindin-D9k in female reproductive tissues: involvement of steroids and endocrine disruptors. Reprod. Biol. Endocrinol. 3, 66. Christakos, S., Barletta, F., Huening, M., Dhawan, P., Liu, Y., Porta, A., Peng, X., 2003. Vitamin D target proteins: function and regulation. J. Cell. Biochem. 88, 238-244. Christakos, S., Beck, J.D., Hyllner, S.J., 1997. Calbindin-D28k. In: Fekdman, D., Glorieux, Pike (Eds.), Vitamin D, Academic Press, San Diego, CA, pp. 209-221 Corradino, R.A., 1993. Calbindin D28K regulation in precociously matured chick egg shell gland in vitro. Gen. Comp. Endocrinol. 91, 158-166. Corradino, R.A., Smith, C.A., Krook, L.P., Fullmer, C.S., 1993. Tissue-specific regulation of shell gland calbindin D28K biosynthesis by estradiol in precociously matured, vitamin D-depleted chicks. Endocrinology 132, 193-198. Corradino, R.A., Wasserman, R.H., Pubols, M.H., Chang, S.I., 1968. Vitamin D 3 induction of a calcium-binding protein in the uterus of the laying hen. Arch. Biochem. Biophys. 125, 378-380. Coty, W.A., 1980. A specific, high affinity binding protein for 1,25-dihydroxyvitamin D in the chick oviduct shell gland. Arch. Biochem. Biophys. 93, 285-292. Coty, W.A., Mc Conkey, C.L., Jr., 1982. A high-affinity calcium-stimulated ATPase activity in the hen oviduct shell gland. Arch. Biochem. Biophys. 219, 444-453. Cunningham, J., Bikle, D.D., Avioli, L.V., 1984. Acute, but not chronic, metabolic acidosis disturbs 25hydroxyvitamin D3 metabolism. Kidney Int. 25, 47-52. Cunningham, J., Bikle, D.D., Avioli, L.V., 1987. Effect of dietary acid and calcium on 25-hydroxyvitamin D metabolism by chick kidney. J. Bone Miner. Res. 2, 289-296. Dacke, C.G., 1976. Parathyroid hormone and eggshell calcification in Japanese quail. J. Endocrinol. 71, 239243. Dacke, C.G., 2000. The parathyroids, calcitonin, and vitamin D. In: Whittow, G.C. (Ed.) Sturkie’s Avian Physiology, Academic Press, New York, pp. 473-488 Dacke, C.G., Arkle, S., Cook, D.J., Wormstone, I.M., Jones, S., Zaidi, M., Bascal, Z.A., 1993a. Medullary bone and avian calcium regulation. J. Exp. Biol. 184, 63-88. Dacke, C.G., Arkle, S., Cook, D.J., Wormstone, I.M., Jones, S., Zaidi, M., Bascal, Z.A., 1993b. Medullary Bone and Avian Calcium Regulation. J. Exp. Biol. 184, 63-88. Davis, W.L., Jones, R.G., Farmer, G.R., Matthews, J.L., Martin, J.H., Bridges, G., 1987. Electron microscopic cytochemical localization of a basolateral calcium adenosine triphosphatase in vitamin D replete chick enterocytes. Anat. Rec. 219, 384-393. Delorme, A.C., Danan, J.L., Acker, M.G., Ripoche, M.A., Mathieu, H., 1983. In rat uterus 17 beta-estradiol stimulates a calcium-binding protein similar to the duodenal vitamin D-dependent calcium-binding protein. Endocrinology 113, 1340-1347. DeLuca, H.F., 2004. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 80, 1689S-1696S. DeLuca, H.F., Nakada, M., Tanaka, Y., Sicinski, R., Phelps, M., 1988. The plasma binding protein for vitamin D is a site of discrimination against vitamin D-2 compounds by the chick. Biochim. Biophys. Acta 965, 16-21. Dick, I.M., Liu, J., Glendenning, P., Prince, R.L., 2003. Estrogen and androgen regulation of plasma membrane calcium pump activity in immortalized distal tubule kidney cells. Mol. Cell. Endocrinol. 212, 11-18. 33 Doi, O., Takai, T., Nakamura, T., Tanabe, Y., 1980. Changes in the pituitary and plasma LH, plasma and follicular progesterone and estradiol, and plasma testosterone and estrone concentrations during the ovulatory cycle of the quail (Coturnix coturnix japonica). Gen. Comp. Endocrinol. 41, 156-163. Drewe, J., Dietsch, P., Keck, E., 1988. Effect of vitamin D status on the activity of carbonic anhydrase in chicken epiphysis and kidney. Calcif. Tissue Int. 43, 26-32. Dulce, H.J., Siegmund, P., Koerber, F., Schuette, E., 1960. [On the biochemistry of the dissolution of bone. 3. On the presence of carbonic anhydrase in bones.]. Hoppe. Seylers Z. Physiol. Chem. 320, 163-167. Dusso, A.S., Brown, A.J., Slatopolsky, E., 2005. Vitamin D. Am. J. Physiol. Renal Physiol. 289, F8-28. Eastin, W.C., Jr., Spaziani, E., 1978. On the mechanism of calcium secretion in the avian shell gland (uterus). Biol. Reprod. 19, 505-518. Edelstein, S., Harell, A., Bar, A., Hurwitz, S., 1975. The functional metabolism of vitamin D in chicks fed lowcalcium and low-phosphorus diets. Biochim. Biophys. Acta 385, 438-442. Edwards, H.M., 2000. Nutrition and skeletal problems in poultry. Poult. Sci. 79, 1018-1023. Ehrenspeck, G., Schraer, H., Schraer, R., 1971. Calcium transfer across isolated avian shell gland. Am. J. Physiol. 220, 967-972. Elaroussi, M.A., Forte, L.R., Eber, S.L., Biellier, H.V., 1994. Calcium homeostasis in the laying hen .1. Age and dietary calcium effects. Poult. Sci. 73, 1581-1589. Eliam, M.C., Basle, M., Bouizar, Z., Bielakoff, J., Moukhtar, M., de Vernejoul, M.C., 1988. Influence of blood calcium on calcitonin receptors in isolated chick osteoclasts. J. Endocrinol. 119, 243-248. Esbaugh, A.J., Tufts, B.L., 2006. The structure and function of carbonic anhydrase isozymes in the respiratory system of vertebrates. Respir. Physiol. Neurobiol. 154, 185-198. Feher, J.J., 1984. Measurement of facilitated calcium diffusion by soluble calcium-binding protein. Biochem. Biophys. Acta .773, 91-98. Feher, J.J., Fullmer, C.S., Wasserman, R.H., 1992. Role of Facilitated Diffusion of Calcium by Calbindin in Intestinal Calcium Absorption. Am. J. Physiol. 262, C517-C526. Findlay, D.M., Sexton, P.M., 2004. Calcitonin. Growth Factors 22, 217-224. Friedlander, E.J., Henry, H.L., Norman, A.W., 1977. Studies on the mode of action of calciferol. Effects of dietary calcium and phosphorus on the relationship between the 25-hydroxyvitamin D3-1 alphahydroxylase and production of chick intestinal calcium binding protein. J. Biol. Chem. 252, 8677-8683. Frost, T.J., Roland, D.A., Untawale, G.G., 1990. Influence of vitamin D3, 1 alpha-hydroxyvitamin D3, and 1,25dihydroxyvitamin D3 on eggshell quality, tibia strength, and various production parameters in commercial laying hens. Poult. Sci. 69, 2008-2016. Fujita, H., Sugimoto, K., Inatomi, S., Maeda, T., Osanai, M., Uchiyama, Y., Yamamoto, Y., Wada, T., Kojima, T., Yokozaki, H., Yamashita, T., Kato, S., Sawada, N., Chiba, H., 2008. Tight Junction proteins claudin-2 and -12 are critical for vitamin D-dependent Ca2+ absorption between enterocytes. Mol. Biol. Cell 19, 1912-1921 Gabriella, M.G., Menghi, G., 1994. The integrative segment of the quail Coturnix coturnix japonica. Occurrence and distribution of carbonic anhydrase and complex carbohydrates. J Anat 185 ( Pt 2), 405-614. Gabrielli, M.G., Vincenzetti, S., Vita, A., Menghi, G., 1998. Immunohistochemical localization of carbonic anhydrase isoenzymes II and III in quail kidney. Histochem. J. 30, 489-497. Gay, C.V., 1996. Avian bone turnover and the role of bone cells. In: Dacke, C., Danks, J., Caple, I., Flik, G. (Eds.), The comparative endocrinology of calcium regulation, Bourne Press, Great Britain, pp. 113-121 Gay, C.V., Gilman, V.R., Sugiyama, T., 2000. Perspectives on osteoblast and osteoclast function. Poult. Sci. 79, 1005-1008. Gay, C.V., Mueller, W.J., 1974. Carbonic anhydrase and osteoclasts: localization by labeled inhibitor autoradiography. Science 183, 432-434. Gill, R.K., Christakos, S., 1995. Regulation by estrogen through the 5'-flanking region of the mouse calbindinD28K gene. Mol. Endocrinol. 9, 319-326. 34 Glendenning, P., Ratajczak, T., Prince, R.L., Garamszegi, N., Strehler, E.E., 2000. The promoter region of the human PMCA1 gene mediates transcriptional downregulation by 1,25-dihydroxyvitamin D3. Biochem. Biophys. Res. Commun. 277, 722-728. Goto, H., Tagami, M., Ina, S., Arakawa, N., Yamamura, N., Takeishi, M., Kamiyoshi, M., 2002a. Effect of sex steroid hormone on gene expression of calcium-binding protein (CaBP-D28K) in the shell gland. Jap. Poult. Sci., 39, J11-J24. Goto, H., Tagami, M., Mizuno, M., Kameoka, E., Takeishi, M., Yamamura, N., Doi, O., Kamiyoshi, M., 2002b. Plasma calcium concentrations and expression of CaBP-D28K mRNA in the shell gland in relation to calcification in the laying hen. Jap. Poult. Sci., 39, J25-J40. Griffin, H.D., 1992. Manipulation of egg yolk cholesterol - a physiologist's view. World Poult. Sci. J. 48, 101112. Grunder, A.A., 1983. Uterine adenosine triphosphatase in relation to shell quality. Poult. Sci. 62, 512-518. Grunder, A.A., Tsang, C.P., Narbaitz, R., 1990. Effects of vitamin D 3 metabolites on physiological traits of White Leghorn. Poult. Sci. 69, 1204-1208. Guerreiro, P.M., Renfro, J.L., Power, D.M., Canario, A.V., 2007. The parathyroid hormone family of peptides: structure, tissue distribution, regulation, and potential functional roles in calcium and phosphate balance in fish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R679-696. Harrison, J.R., Clark, N.B., 1986. Avian medullary bone in organ culture: effects of vitamin D metabolites on collagen synthesis. Calcif. Tissue Int. 39, 35-43. Hart, L.E., DeLuca, H.F., Yamada, S., Takayama, H., 1984. Hydroxylation of carbon-24 of 25hydroxycholecalciferol is not necessary for normal embryonic development in chickens. J. Nutr. 114, 20592065. Haussler, M.R., Whitfield, G.K., Haussler, C.A., Hsieh, J.C., Thompson, P.D., Selznick, S.H., Dominguez, C.E., Jurutka, P.W., 1998. The nuclear vitamin D receptor: Biological and molecular regulatory properties revealed. J. Bone Miner. Res. 13, 325-349. Henry, H.L., 1997. The 25-hydroxyvitamin D 1 alpha-hydroxylase. In: Feldman, D. (Ed.) Vitamin D, Academic Press Inc, San Diego, CA, pp. 57-68 Henry, H., Midgett, R.J., Norman, A.W., 1974. Regulation of 25-hydroxyvitamin D3-1-hydroxylase in vivo. J. Biol. Chem. 249, 7584-7592. Henry, H.L., Norman, A.W., 1978. Vitamin D: two dihydroxylated metabolites are required for normal chicken egg hatchability. Science 201, 835-837. Henry, H.L., Norman, A.W., Taylor, A.N., Hartenbower, D.L., Coburn, J.W., 1976. Biological activity of 24,25-dihydroxycholecalciferol in chicks and rats. J. Nutr. 106, 724-734. Hoenderop, J.G., Bindels, R.J., 2005a. Epithelial Ca 2+ and Mg2+ channels in health and disease. J. Am. Soc. Nephrol. 16, 15-26. Hoenderop, J.G., Nilius, B., Bindels, R.J., 2005b. Calcium absorption across epithelia. Physiol. Rev. 85, 373422. Hoenderop, J.G.J., Nilius, B., Bindels, R.J.M., 2002. Molecular mechanism of active Ca2+ reabsorption in the distal nephron. Annu. Rev. Physiol. 64, 529-549. Holick, M.F., 2003. Vitamin D: A millenium perspective. J. Cell. Biochem. 88, 296-307. Holmes, R.S., 1977. Purification, molecular properties and ontogeny of carbonic anhydrase isozymes. Evidence for A, B and C isozymes in avian and mammalian tissues. Eur. J. Biochem. 78, 511-520. Howard, A., Legon, S., Walters, J.R., 1993. Human and rat intestinal plasma membrane calcium pump isoforms. Am. J. Physiol. 265, G917-G925. Huang, C.L., Sun, L., Moonga, B.S., Zaidi, M., 2006. Molecular physiology and pharmacology of calcitonin. Cell Mol. Biol. (Noisy le grand) 52, 33-43. Hunziker, W., 1986. The 28-kDa vitamin D-dependent calcium-binding protein has a six domain structure. Proc. Natl. Acad. Sci. USA 83, 7578-7582. 35 Hunziker, W., Walters, M.R., Bishop, J.E., Norman, A.W., 1982. Effect of vitamin D status on the equilibrium between occupied and unoccupied 1,25-dihydroxyvitamin D intestinal receptors in the chick. J. Clin. Invest. 69, 826-833. Hurwitz, S., 1992. The role of vitamin D in poultry bone biology. In: Whithead, C.C. (Ed.) Bone Biology and Skeletal Disorders in Poultry, vol. 23. Carfax, Abingdon, pp. 87-102 Hurwitz, S., 1996. Homeostatic control of plasma calcium concentration. Crit. Rev. Biochem. Mol. Biol. 31, 41100. Hurwitz, S., Bar, A., 1965. Absorption of calcium and phosphorus along the gastrointestinal tract of the laying fowl as influenced by dietary calcium and egg shell formation. J. Nutr. 86, 433-438. Hurwitz, S., Bar, A., 1966. Rate of passage of calcium-45 and yttrium-91 along the intestine, and calcium absorption in the laying fowl. J. Nutr. 89, 311-316. Hurwitz, S., Bar, A., 1968. Activity, concentration, and lumen-blood electrochemical potential difference of calcium in the intestine of the laying hen. J. Nutr. 95, 647-654. Hurwitz, S., Bar, A., 1969. Intestinal calcium absorption in the laying fowl and its importance in calcium homeostasis. Am. J. Clin. Nutr. 22, 391-395. Hurwitz, S., Bar, A., Cohen, I., 1973. Regulation of calcium absorption by fowl intestine. Am. J. Physiol. 225, 150-154. Ieda, T., Saito, N., Ono, T., Shimada, K., 1995. Effects of presence of an egg and calcium deposition in the shell gland on levels of messenger ribonucleic acid of CaBP-D-28K and of vitamin D-3 receptor in the shell gland of the laying hen. Gen. Comp. Endocrinol. 99, 145-151. Ieda, T., Saito, N., Shimada, K., 1999. Effect of low calcium diet on messenger ribonucleic acid levels of calbindin-D28K of intestine and shell gland in laying hens in relation to egg shell quality. Jap. Poult. Sci. 36, 295-303. Ieda, T., Takahashi, T., Saito, N., Yasuoka, T., Kawashima, M., Shimada, K., 2000. Changes in parathyroid hormone-related peptide receptor binding in the shell gland of laying hens (Gallus domesticus) during the oviposition cycle. Gen. Comp. Endocrinol. 117, 182-188. Jande, S.S., Tolnai, S., Lawson, D.E., 1981. Immunohistochemical localization of vitamin D-dependent calcium-binding protein in duodenum, kidney, uterus and cerebellum of chickens. Histochemistry 71, 99116. Johnson, A.I., van Tienhoven, A., 1980. Plasma concentrations of six steroids and LH during the ovulatory cycle of the hen, Gallus domesticus. Biol. Reprod. 23, 386-393. Jones, G., Strugnell, S.A., DeLuca, H.F., 1998. Current understanding of the molecular actions of vitamin D. Physiol. Rev. 78, 1193-1231. Joyner, C.I., Peddie, M.J., Taylor, T.C., 1987. The effect of age on egg production in the domestic hen. Gen. Comp. Endocrinol. 65, 331-336. Kaetzel, D.M., Jr., Soares, J.H., Jr., 1985. Effect of dietary calcium stress on plasma vitamin D3 metabolites in the egg-laying Japanese quail. Poult. Sci. 64, 1121-1127. Kaetzel, D.M.J., Soares, J.H.J., 1984. The effects of dietary cholecalciferol steroids and gonadal hormone implantation on plasma 1,25-dihydroxycholecalciferol and bone calcification in preovulatory and senescent female Japanese quail. Nutr. Res. 4, 621-632. Kenny, A.D., 1976. Vitamin D metabolism: physiological regulation in egg-laying Japanese quail. Am. J. Physiol. 230, 1609-1615. Kong, J., Zhang, Z., Musch, M.W., Ning, G., Sun, J., Hart, J., Bissonnette, M., Li, Y.C., 2008. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol 294, G208-216. Koster, H.P.G., Hartog, A., Vanos, C.H., Bindels, R.J.M., 1995. Calbindin-D(28K) facilitates cytosolic calcium diffusion without interfering with calcium signaling. Cell Calcium 18, 187-196. Kream, B.E., Lichtler, A.C., 1997. Vitamin D regulation of type I collagen synthesis and gene expression in bone. In: Feldman, D. (Ed.) Vitamin D, Academic Press Inc, San Diego, CA pp. 201-207. 36 Kutuzova, G.D., Deluca, H.F., 2004. Gene expression profiles in rat intestine identify pathways for 1,25dihydroxyvitamin D(3) stimulated calcium absorption and clarify its immunomodulatory properties. Arch. Biochem. Biophys. 432, 152-166. Lambers, T.T., Bindels, R.J., Hoenderop, J.G., 2006. Coordinated control of renal Ca2+ handling. Kidney Int. 69, 650-654. Larsson, B., Nemere, I., 2003. Effect of growth and maturation on membrane-initiated actions of 1,25dihydroxyvitamin D(3). I. Calcium transport, receptor kinetics, and signal transduction in intestine of male chickens. Endocrinology 144, 1726-1735. Lawson, D.E.M., 1978. Biochemical responses of the intestine to vitamin D. In: Lawson, D.E.M. (Ed.) Vitamin D, Academic Press, London, pp. 167-200 Lee, G.S., Jeung, E.B., 2007. Uterine TRPV6 expression during estrous cycle and pregnancy in a mouse model. Am. J. Physiol. Endocrinol. Metab. 293, E132-E138. Lidor, C., Dekel, S., Meyer, M.S., Blaugrund, E., Hallel, T., Edelstein, S., 1990. Biochemical and biomechanical properties of avian callus after local administration of dihydroxylated vitamin D metabolites. J. Bone Joint Surg. Br. 72, 137-140. Lippiello, L., Wasserman, R.H., 1975. Fluorescent antibody localization of the vitamin D-dependent calciumbinding protein in the oviduct of the laying hen. J. Histochem. Cytochem. 23, 111-116. Lomri, A., Baron, R., 1992. 1 alpha 25-Dihydroxyvitamin-D3 Regulates the Transcription of Carbonic Anhydrase-II messenger RNA in Avian Myelomonocytes. Proc, Natl. Acad. Sci. USA 89, 4688-4692. Lundholm, C.E., 1982. Effect of p-p'-DDE administered in vivo and in vitro on Ca2+ binding and Ca2+-Mg2+ATPase activity in egg shell gland mucose of ducks. Acta Pharmacol. Toxicol. 50, 121-129. Lundholm, C.E., 1990. The eggshell thinning action of acetazolamide; Relation to the binding of Ca2+ and carbonic anhydrase activity of shell gland homogenate. Comp. Biochem. Physiol. 95 C, 85-89. Lundholm, C.E., 1997. DDE-induced eggshell thinning in birds: Effects of p,p'-DDE on the calcium and prostaglandin metabolism of the eggshell gland. Comp. Biochem. Physiol. C 118, 113-128. Lytton, J., 2007. Na+/ Ca2+ exchangers: three mammalian gene families control Ca2+ transport. Biochem. J. 406, 365-382. Marche, P., Delorme, A., Cuisinier Gleizes, P., 1978. Intestinal and placental calcium-binding proteins in vitamin D-deprived or -supplemented rats. Life Sci. 23, 2555-2561. Mayel-Afshar, S., Lane, S.M., Lawson, D.E.M., 1988. Relationships between the levels of calbindin synthesis and calbindin mRNA in chick intestine. Quantitation of calbindin mRNA. J. Biol. Chem. 263, 43554361. McCormick, C.C., 2002. Passive diffusion does not play a major role in the absorption of dietary calcium in normal adults. J. Nutr. 132, 3428-3430. Melancon, M.J., Jr., DeLuca, H.F., 1970. Vitamin D stimulation of calcium-dependent adenosine triphosphatase in chick intestinal brush borders. Biochemistry 9, 1658-1664. Meyer, J., Fullmer, C.S., Wasserman, R.H., Komm, B.S., Haussler, M.R., 1992. Dietary restriction of calcium, phosphorus, and vitamin D elicits differential regulation of the mRNAs for avian intestinal calbindinD28k and the 1,25-dihydroxyvitamin D3 receptor. J. Bone Miner. Res. 7, 441-448. Miller, S.C., 1992. Calcium homeostasis and mineral turnover in the laying hen. In: Whithead, C.C. (Ed.) Bone biology and skeletal disorders in poultry, vol. 23. Carfax, Abingdon, pp. 103-116 Montecuccoli, G., Bar, A., Risenfeld, G., Hurwitz, S., 1977a. The response of 25-hydroxycholecalciferol-1hydroxylase activity, intestinal calcium absorption, and calcium-binding protein to phosphate deficiency in chicks. Comp. Biochem. Physiol. A 57, 331-334. Montecuccoli, G., Hurwitz, S., Cohen, A., Bar, A., 1977b. The role of 25-hydroxycholecalciferol-1-hydroxylase in the responses of calcium absorption to the reproductive activity in birds. Comp. Biochem. Physiol. A 57, 335-339. Morrissey, R.L., Wasserman, R.H., 1971. Calcium absorption and calcium-binding protein in chicks on differing calcium and phosphorus intakes. Am. J. Physiol. 220, 1509-1515. 37 Navickis, R.J., Dial, O.K., Katzenellenbogen, B.S., Nalbandov, A.V., 1979a. Effects of gonadal hormones on calcium-binding protein in chick duodenum. Am. J. Physiol. 237, E408-E417. Navickis, R.J., Katzenellenbogen, B.S., Nalbandov, A.V., 1979b. Effects of the sex steroid hormones and vitamin D3 on calcium-binding proteins in the chick shell gland. Biol. Reprod. 21, 1153-1162. Nemere, I., 1992. Vesicular Calcium Transport in Chick Intestine. J. Nutr. 122, 657-661. Nemere, I., Norman, A.W., 1990. Transcaltachia, vesicular calcium transport, and microtubule-associated calbindin-D28K: emerging views of 1,25-dihydroxyvitamin D3-mediated intestinal calcium absorption. Miner. Electrolyte Metab. 16, 109-114. Nemere, I., Yoshimoto, Y., Norman, A.W., 1984. Calcium transport in perfused duodena from normal chicks: enhancement within fourteen minutes of exposure to 1,25-dihydroxyvitamin D3. Endocrinology 115, 14761483. Newbrey, J.W., Truitt, S.T., Roland, D.A., Frost, T.J., Untawale, G.G., 1992. Bone histomorphometry in 1,25(OH)2D3- and vitamin D3-treated aged laying hens. Avian Dis. 36, 700-706. Newman, S., Leeson, S., 1999. The effect of dietary supplementation with 1,25-dihydroxycholecalciferol or vitamin C on the characteristics of the tibia of older laying hens. Poult. Sci. 78, 85-90. Niemeyer, B.A., 2005. Structure-function analysis of TRPV channels. Naunyn Schmiedebergs Arch. Pharmacol. 371, 285-294. Nijenhuis, T., Hoenderop, J.G., Bindels, R.J., 2005. TRPV5 and TRPV6 in Ca 2+ (re)absorption: regulating Ca2+ entry at the gate. Pflugers Arch. 451, 181-192. Norman, A.W., 1995. The vitamin D endocrine system: Manipulation of structure-function relationships to provide opportunities for development of new cancer chemopreventive and immunosuppressive agents. J. Cell. Biochem. 218-225. Norman, A.W., 2006. Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology 147, 5542-5548. Norman, A.W., Hurwitz, S., 1993. The role of the vitamin D endocrine system in avian bone biology. J. Nutr. 123, 310-316. Norman, A.W., Okamura, W.H., Bishop, J.E., Henry, H.L., 2002. Update on biological actions of 1alpha,25(OH)2-vitamin D3 (rapid effects) and 24R,25(OH) 2-vitamin D3. Mol. Cell. Endocrinol. 197, 1-13. Nys, Y., Baker, K., Bouillon, R., Vanbaelen, H., Lawson, D.E.M., 1992a. Regulation of calbindin-D-28K and its messenger RNA in the intestine of the domestic hen. Gen. Comp. Endocrinol. 86, 460-468. Nys, Y., Baker, K., Lawson, D.E., 1992b. Estrogen and a calcium flux dependent factor modulate the calbindin gene expression in the uterus of laying hens. Gen. Comp. Endocrinol. 87, 87-94. Nys, Y., de Laage, X., 1984a. Effects of suppression of eggshell calcification and of 1,25(OH)2D3 on Mg 2+, Ca2+ and Mg2+ HCO3ATPase, alkaline phosphatase, carbonic anhydrase and CaBP levels--I. The laying hen uterus. Comp. Biochem. Physiol. A 78, 833-838. Nys, Y., de Laage, X., 1984b. Effects of suppression of eggshell calcification and of 1,25(OH) 2D3 on Mg2+, Ca2+ and Mg2+HCO3 ATPase, alkaline phosphatase, carbonic anhydrase and CaBP levels--II. The laying hen intestine. Comp. Biochem. Physiol. A 78, 839-844. Nys, Y., Mayel Afshar, S., Bouillon, R., Van Baelen, H., Lawson, D.E., 1989. Increases in calbindin D 28K mRNA in the uterus of the domestic fowl induced by sexual maturity and shell formation. Gen. Comp. Endocrinol. 76, 322-329. Nys, Y., N'Guyen, T.M., Garabedian, M., 1984c. Involvement of 1,25-dihydroxycholecalciferol in the shortand long-term increase of intestinal calcium absorption in laying hens: stimulation by gonadal hormones is partly independent of 1,25-dihydroxycholecalciferol. Gen. Comp. Endocrinol. 54, 59-68. Nys, Y., N'Guyen, T.M., Williams, J., Etches, R.J., 1986a. Blood levels of ionized calcium, inorganic phosphorus, 1,25-dihydroxycholecalciferol and gonadal hormones in hens laying hard-shelled or shellless eggs. J. Endocrinol. 111, 151-157. Nys, Y., Parkes, C.O., Thomasset, M., 1986b. Effects of suppression and resumption of shell formation and parathyroid hormone on uterine calcium-binding protein, carbonic anhydrase activity, and intestinal calcium absorption in hens. Gen. Comp. Endocrinol. 64, 293-299. 38 Nys, Y., Vanbaelen, H., Bouillon, R., 1992c. Plasma 1,25 Dihydroxycholecalciferol and Its Free Index Are Potentiated by Ovulation Dependent Factors and Shell Formation Induced Hypocalcemia in the Laying Hens. Domest. Anim. Endocrinol. 9, 37-47. Ohira, H., Yoshimura, Y., Tamura, T., 1998. Increase in calcium binding protein-D28K contents in the shell gland by an injection of 1,25-dihydroxyvitamin D3 into the shell gland lumen in laying hens. Jap Poult. Sci. 35, 99-107. Ohyama, Y., Yamasaki, T., 2004. Eight cytochrome P450s catalyze vitamin D metabolism. Front. Biosci. 9, 3007-3018. Omdahl, J.L., Morris, H.A., May, B.K., 2002. Hydroxylase enzymes of the vitamin D pathway: expression, function, and regulation. Annu. Rev. Nutr. 22, 139-166. Ornoy, A., Goodwin, D., Noff, D., Edelstein, S., 1978. 24, 25-dihydroxyvitamin D is a metabolite of vitamin D essential for bone formation. Nature 276, 517-519. Pannabecker, T.L., Chandler, J.S., Wasserman, R.H., 1995. Vitamin-D-dependent transcriptional regulation of the intestinal plasma membrane calcium pump. Biochem. Biophys. Res. Commun. 213, 499-505. Pearson, T.W., Pryor, T.J., Goldner, A.M., 1977. Calcium transport across avian uterus. III. Comparison of laying and nonlaying birds. Am. J. Physiol. 232, E437-E443. Peng, J.B., Chen, X.Z., Berger, U.V., Vassilev, P.M., Tsukaguchi, H., Brown, E.M., Hediger, M.A., 1999. Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J. Biol. Chem. 274, 22739-22746. Pike, J.W., 1997. The vitamin D receptor and its gene. In: Feldman, D. (Ed.) Vitamin D, Academic Press Inc, San Diego, CA 92101-4495, pp. 105-125 Pike, J.W., Alvarado, R.H., 1975. Ca2+-Mg-2+-activated ATPase in the shell gland of japanese quail (Coturnix coturnix japonica). Comp. Biochem. Physiol. B 51, 119-125. Potts, J.T., 2005. Parathyroid hormone: past and present. J. Endocrinol. 187, 311-325. Potts, J.T., Gardella, T.J., 2007. Progress, paradox, and potential: parathyroid hormone research over five decades .Ann. N. Y. Acad. Sci. 1117, 196-208. Prosser, D.E., Jones, G., 2004. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem. Sci. 29, 664-673. Purkerson, J.M., Schwartz, G.J., 2007. The role of carbonic anhydrases in renal physiology. Kidney Int 71, 103115. Putkey, J.A., Spielvogel, A.M., Sauerheber, R.D., Dunlap, C.S., Norman, A.W., 1982. Vitamin D-mediated intestinal calcium transport. Effects of essential fatty acid deficiency and spin label studies of enterocyte membrane lipid fluidity.Biochim. Biophys. Acta 688, 177-190. Qin, X., Klandorf, H., 1993a. Effect of estrogen in relation to dietary vitamin D 3 and calcium on activity of intestinal alkaline phosphatase and Ca-ATPase in immature chicks. Gen. Comp. Endocrinol. 90, 318-327. Qin, X., Klandorf, H., Porter, D.W., Holt, S.B., Martin, W.G., 1993b. Estrogen enhancement of calcium, magnesium, and calcium-magnesium stimulated adenosine triphosphatase activity in the chick shell gland. Gen. Comp. Endocrinol. 89, 4-10. Quelo, I., Kahlen, J.P., Rascle, A., Jurdic, P., Carlberg, C., 1994. Identification and characterization of a vitamin D3 response element of chicken carbonic anhydrase-II. DNA Cell Biol. 13, 1181-1187. Rabon, H.W., Jr., Roland, D.A., Sr., Clark, A.J., 1991. Uterine calcium-binding protein activity of nonlaying hens and hens laying hard-shelled or shell-less eggs. Poult. Sci. 70, 2280-2283. Ramasamy, I., 2006. Recent advances in physiological calcium homeostasis. Clin. Chem. Lab. Med. 44, 237273. Rasmussen, H., Matsumoto, T., Fontaine, O., Goodman, D.B., 1982. Role of changes in membrane lipid structure in the action of 1,25-dihydroxyvitamin D3. Fed. Proc. 41, 72-77. Reddy, G.S., Jones, G., Kooh, S.W., Fraser, D., 1982. Inhibition of 25-hydroxyvitamin D3-1-hydroxylase by chronic metabolic acidosis. Am. J. Physiol. 243, E265-271. 39 Ribovich, M.L., DeLuca, H.F., 1975. The influence of dietary calcium and phosphorus on intestinal calcium transport in rats given vitamin D metabolites. Arch. Biochem. Biophys. 170, 529-535. Ro, H.K., Tembe, V., Krug, T., Yang, P.Y., Bushinsky, D.A., Favus, M.J., 1990. Acidosis inhibits 1,25(OH)2D3 but not cAMP production in response to parathyroid hormone in the rat. J. Bone Miner. Res. 5, 273-278. Rosenberg, J., Hurwitz, S., Bar, A., 1986. Regulation of kidney calcium-binding protein in the bird (Gallus domesticus). Comp. Biochem. Physiol. A 83, 277-281. Sasayama, Y., 1999. Hormonal control of Ca homeostasis in lower vertebrates: Considering the evolution. Zool. Sci. 16, 857-869. Sauveur, B., Garabedian, M., Fellot, C., Mongin, P., Balsan, S., 1977. The effect of induced metabolic acidosis on vitamin D3 metabolism in rachitic chicks. Calcif. Tissue Res. 23, 121-124. Schneeberger, E.E., Lynch, R.D., 2004. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol 286, C1213-1228. Schraer, H., Schraer, R., 1970. Calcium translocation in the avian shell gland. Ann. Biol. Anim. Biochim. Biophys. 10, 229-244. Sedrani, S., Taylor, T.G., 1977. Metabolism of 25-hydroxycholecalciferol in Japanese quail in relation to reproduction. J. Endocrinol. 72, 405-406. Singh, R., Joyner, C.J., Peddie, M.J., Taylor, T.G., 1986. Changes in the concentrations of parathyroid hormone and ionic calcium in the plasma of laying hens during the egg cycle in relation to dietary deficiencies of calcium and vitamin D. Gen. Comp. Endocrinol. 61, 20-28. Soares, J.H., Kerr, J.M., Gray, R.W., 1995. 25-hydroxycholecalciferol in poultry nutrition. Poult. Sci. 74, 19191934. Soares, J.H., Ottinger, M.A., Buss, E.G., 1988. Potential role of 1,25 dihydroxycholecalciferol in shell calcification. Poult. Sci. 67, 1322-1328. Sommerville, B.A., Scanes, C.G., Swaminathan, R., Care, A.D., Harvey, S., Chadwick, A., 1989. Effect of estrogen on calcium homeostasis and pituitary hormones in the growing chick. Gen. Comp. Endocrinol. 76, 261-266. Spanos, E., Pike, J.W., Haussler, M.R., Colston, K.W., Evans, I.M.A., Goldner, A.M., McCain, T.A., McIntyre, I., 1976. Circulating 1 alpha,25-dihydroxyvitamin D in the chicken: enhancement of injection of prolactin and during egg laying. Life Sci. 19, 1751-1756. Stokes, D.L., Green, N.M., 2003. Structure and function of the calcium pump. Annu. Rev. Biophys. Biomol. Struct. 32, 445-468. Strehler, E.E., Caride, A.J., Filoteo, A.G., Xiong, Y., Penniston, J.T., Enyedi, A., 2007. Plasma membrane Ca2+ ATPases as dynamic regulators of cellular calcium handling. Ann. N. Y. Acad. Sci. 1099, 226-236. Striem, S., 1990. Regulation of calcium-binding protein (calbindin 28KDA) synthesis in the avian uterus. Master Sci. degree, Hebrew university, Jerusalem, Israel. Striem, S., Bar, A., 1989. Vitamin D metabolism and expression in estrogen-treated female birds. Abstracts; The XXI European Symposium on Calcified Tissues. Calcif. Tissue. Int. 44, s-26. Striem, S., Bar, A., 1991. Modulation of quail intestinal and egg shell gland calbindin (Mr 28,000) gene expression by vitamin D3, 1,25-dihydroxyvitamin D3 and egg laying. Mol. Cell. Endocrinol. 75, 169-177. Strittmatter, C.F., 1972. Phosphatase activities of chicken liver and duodenum: characteristics, levels during development, and hydrocortisone-induced changes. Biochim. Biophys. Acta 284, 183-195. Sugiyama, T., Kusuhara, S., 2001. Avian calcium metabolism and bone function. Asian Aust. J. Anim. Sci. 14, 82-90. Sugiyama, T., Kikuchi, H., Hiyama, S., Nishizawa, K., Kusuhara, S., 2007. Expression and localisation of calbindin D28k in all intestinal segments of the laying hen. Br. Poult. Sci. 48, 233-238. Takahashi, N., Abe, E., Tanabe, R., Suda, T., 1980. A high-affinity cytosol binding protein for 1 alpha,25dihydroxycholecalciferol in the uterus of Japanese quail. Biochem. J. 190, 513-518. 40 Tanaka, Y., Castillo, L., Deluca, H.F., 1976. Control of renal vitamin D hydroxylases in birds by sex hormones. Proc. Natl. Acad. Sci. USA 73, 2701-2705. Taylor, A.N., Wasserman, R.H., 1967. Vitamin D3-induced calcium-binding protein: partial purification, electrophoretic visualization, and tissue distribution. Arch. Biochem. Biophys. 119, 536-540. Taylor, A.N., Wasserman, R.H., 1969. Correlations between the vitamin D-induced calcium binding protein and intestinal absorption of calcium. Fed. Proc. 28, 1834-1838. Taylor, A.N., Wasserman, R.H., 1972. Vitamin D-induced calcium binding protein: comparative aspects in kidney and intestine. Am J Physiol. 223, 110-114. Thomasset, M., 1997. Calbindin-D9k. In: Feldman, D., Glorieux, Pike (Eds.), Vitamin D, Academic Press, pp. 223-232 Thomasset, M., Desplan, C., Moukhtar, M., Mathieu, H., 1981. Primary Translation product of mRNA coding for rat duodenal vitamin D-dependent calcium-binding protein. FEBS Lett. 134, 178-182. Tang, V.W., Goodenough, D.A., 2003. Paracellular ion channel at the tight junction. Biophys. J. 84, 16601673. van Abel, M., Hoenderop, J.G., Bindels, R.J., 2005. The epithelial calcium channels TRPV5 and TRPV6: regulation and implications for disease. Naunyn Schmiedebergs Arch. Pharmacol. 371, 295-306. Van Cromphaut, S.J., Rummens, K., Stockmans, I., Van Herck, E., Dijcks, F.A., Ederveen, A.G., Carmeliet, P., Verhaeghe, J., Bouillon, R., Carmeliet, G., 2003. Intestinal calcium transporter genes are upregulated by estrogens and the reproductive cycle through vitamin D receptor-independent mechanisms. J. Bone Miner. Res. 18, 1725-1736. van de Graaf, S.F., Bindels, R.J., Hoenderop, J.G., 2007. Physiology of epithelial Ca 2+ and Mg2+ transport. Rev. Physiol. Biochem. Pharmacol. 158, 77-160. van de Velde, J.P., Loveridge, N., Vermeiden, J.P.W., 1984. Parathyroid hormone responses to calcium stress during eggshell calcification. Endocrinology 115, 1901-1904. van der Eerden, B.C., Hoenderop, J.G., de Vries, T.J., Schoenmaker, T., Buurman, C.J., Uitterlinden, A.G., Pols, H.A., Bindels, R.J., van Leeuwen, J.P., 2005. The epithelial Ca2 channel TRPV5 is essential for proper osteoclastic bone resorption. Proc. Natl. Acad. Sci. USA 102, 17507-17512. Van-Abel, M., Hoenderop, J.G.J., van-der-Kemp, A., Van-Leeuwen, J.P.T.M., Bindels, R.J.M., 2003. Regulation of the epithelial Ca2 channels in small intestine as studied by quantitative mRNA detection. Am. J. Physiol. 285, G78-G85. Van Itallie, C.M., Anderson, J.M., 2006. Claudins and epithelial paracellular transport. Annu. Rev. Physiol. 68, 403-429. Venkatachalam, K., Montell, C., 2007. TRP channels. Annu. Rev. Biochem. 76, 387-417. Walters, M.R., 1997. Other vitamin D target tissues: Vitamin D actions in cardiovascular tissue and muscle, endocrine and reproductive tissues, and liver and lung. In: Feldman, D. (Ed.) Vitamin D, Academic Press Inc, San Diego, CA, pp. 463-482 Walzem, R.L., 1996. Lipoproteins and the laying hen: form follows function. Poultry and Avian Biol. Rev. 7, 31-64. Walzem, R.L., Hansen, R.J., Williams, D.L., Hamilton, R.L., 1999. Estrogen induction of VLDLy assembly in egg-laying hens. J. Nutr. 129, 467S-472S. Wasserman, R.H., 1997. Vitamin D and the intestinal absorption of calcium and phosphorus. In: Feldman, D. (Ed.) Vitamin D, Academic Press Inc, San Diego, CA, pp. 259-273 Wasserman, R.H., 2004. Vitamin D and the dual processes of intestinal calcium absorption. J. Nutr. 134, 31373139. Wasserman, R.H., Smith, C.A., Brindak, M.E., De Talamoni, N., Fullmer, C.S., Penniston, J.T., Kumar, R., 1992. Vitamin D and mineral deficiencies increase the plasma membrane calcium pump of chicken intestine. Gastroenterology 102, 886-894. 41 Wasserman, R.H., Smith, C.A., Smith, C.M., Brindak, M.E., Fullmer, C.S., Krook, L., Penniston, J.T., Kumar, R., 1991. Immunohistochemical localization of a calcium pump and calbindin-D28k in the oviduct of the laying hen. Histochemistry 96, 413-418. Wasserman, R.H., Taylor, A.N., 1966. Vitamin D-induced calcium-binding protein in chick intestinal mucosa. Science 152, 791-793. Wasserman, R.H., Taylor, A.N., 1968. Vitamin D-dependent calcium-binding protein. Response to some physiological and nutritional variables. J. Biol. Chem. 243, 3987-3993. Watanabe, E., Kobayashi, S., Terashima, Y., Itoh, H., 1989. Adenosine triphosphatase in the uterus and duodenum of chicken hens during eggshell formation. Poult. Sci. 68, 564-568. Weber, K., Erben, R.G., Rump, A., Adamski, J., 2001. Gene structure and regulation of the murine epithelial calcium channels ECaC1 and 2. Biochem. Biophys. Res. Commun. 289, 1287-1294. Whitehead, C.C., 1998. A review of nutritional and metabolic factors involved in dyschondroplasia in poultry. J. Appl. Anim. Res. 13, 1-16. Whitehead, C.C., 2004. Overview of bone biology in the egg-laying hen. Poult. Sci. 83, 193-199. Wu, J.C.Y., Smith, M.W., Turvey, A., Keable, S.J., Colston, K.W., 1994. Differential regulation of vitamin d receptor and intestinal calcium transport occurring during sexual maturation in the fowl (Gallus domesticus). Comp. Biochem. Physiol. A 109, 713-720. Yamamoto, T., Ozawa, H., Nagai, H., 1985. Histochemical studies of Ca-ATPase, succinate and NAD+dependent isocitrate dehydrogenases in the shell gland of laying Japanese quails: with special reference to calcium-transporting cells. Histochemistry 83, 221-226. Yasuoka, T., Kawashima, M., Takahashi, T., Iwata, A., Oka, N., Tanaka, K., 1996. Changes in parathyroid hormone receptor binding affinity during egg laying: Implications for calcium homeostasis in chicken. J. Bone Miner. Res. 11, 1913-1920. Yosefi, S., Braw-Tal, R., Bar, A., 2003. Intestinal and eggshell calbindin and bone ash as influenced by age of the laying hen and molting. Comp. Biochem. Physiol. A 136, 673-682. Yoshimura, Y., Ohira, H., Tamura, T., 1997. Immunocytochemical localization of vitamin D receptors in the shell gland of immature, laying, and molting hens. Gen. Comp. Endocrinol. 108, 282-289. Young, H.S., Stokes, D.L., 2004. The mechanics of calcium transport. J. Membr. Biol. 198, 55-63. Zaidi, M., Bascal, Z.A., Adebanjo, O.A., Arkle, S., Dacke, C.G., 1996. Ca 2+-sensing receptors in avian osteoclasts. In: Dacke, C., Danks, J., Caple, I., Flik, G. (Eds.), The comparative endocrinology of calcium regulation, Bourne Press, Great Britain, pp. 123-130 Zelinski, J.M., Sykes, D.E., Weiser, M.M., 1991. The effect of vitamin D on rat intestinal plasma membrane CA-pump mRNA. Biochem. Biophys. Res. Commun. 179, 749-755. Zhu, Y., Goff, J.P., Reinhardt, T.A., Horst, R.L., 1998. Pregnancy and lactation increase vitamin D-dependent intestinal membrane calcium adenosine triphosphatase and calcium binding protein messenger ribonucleic acid expression. Endocrinology 139, 3520-3524.