FatsVitaminsReview
advertisement

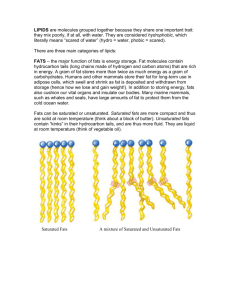
Fats and Vitamins Review 1. Fats and oils can be described as esters of glycerol, C3H8O3. (a) (i) Draw the structure of glycerol. (1) (ii) Glycerol can react with three molecules of stearic acid, C17H35COOH, to form a triglyceride. Deduce the number of carbon atoms in one molecule of this triglyceride. ………………………………………………………………………………… (1) (iii) A triglyceride is also formed in the reaction between glycerol and three molecules of oleic acid, C17H33COOH. State and explain which of the two triglycerides (the one formed from stearic acid or the one formed from oleic acid) has the higher melting point. ………………………………………………………………………………… ………………………………………………………………………………… ………………………………………………………………………………… ………………………………………………………………………………… ………………………………………………………………………………… (3) (b) An oil sample containing 0.0100 mol of oil was found to react with 7.61 g of iodine, I2. Determine the number of C=C double bonds present in each molecule of the oil. ………………………………………………………………………………………. ………………………………………………………………………………………. ………………………………………………………………………………………. ………………………………………………………………………………………. (2) (Total 7 marks) 1 2. Linoleic acid, C17H31COOH, (Mr = 280) and stearic acid, C17H35COOH, (Mr = 284) both contain eighteen carbon atoms and have similar molar masses. (a) Explain why the melting point of linoleic acid is considerably lower than the melting point of stearic acid. ...................................................................................................................................... ...................................................................................................................................... ...................................................................................................................................... ...................................................................................................................................... ...................................................................................................................................... ...................................................................................................................................... (3) (b) Determine the maximum mass of iodine, I2, (Mr = 254) that can add to (i) 100 g of stearic acid: ................................................... ......................................................................... ......................................................................... (1) (ii) 100 g of linoleic acid: .................................................. ......................................................................... ......................................................................... (2) (c) (i) Draw the simplified structural formula of a fat containing one stearic acid and two linoleic acid residues. (1) (ii) Give the formulas of the products formed when this fat is hydrolyzed by sodium hydroxide. (1) (Total 8 marks) 2 3. The structural formulas of cholesterol and testosterone are shown in Table 22 of the Data Booklet. (a) Identify the class of compound to which cholesterol and testosterone belong. ..................................................................................................................................... (1) (b) State the names of two functional groups present in both cholesterol and testosterone. ..................................................................................................................................... ..................................................................................................................................... (2) (c) Cholesterol and testosterone both contain a five-membered ring as part of their structures. Deduce the total number of hydrogen atoms joined directly to the carbon atoms in this ring. ..................................................................................................................................... ..................................................................................................................................... (1) (Total 4 marks) 4. (a) A brand of vegetable fat consists of 88 % unsaturated fats and 12 % saturated fats. State the major structural difference between unsaturated and saturated fats. ..................................................................................................................................... ..................................................................................................................................... (1) (b) Linoleic acid, CH3(CH2)4CH==CHCH2CH ==CH(CH2)7COOH, and palmitic acid, CH3(CH2)14COOH, are components of vegetable fat. Explain why palmitic acid has the higher melting point. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (3) 3 (c) The energy content of a vegetable oil was determined using a calorimeter. A 5.00 g sample of the oil was completely combusted in a calorimeter containing 1 000 g of water at an initial temperature of 18.0 °C. On complete combustion of the oil, the temperature of the water rose to 65.3° C. Calculate the calorific value of the oil in kJ g−1. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (4) (d) List two functions of fats in the human body. ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... ..................................................................................................................................... (2) (Total 10 marks) 4 5. By referring to Table 22 of the Data Booklet, identify one vitamin that is water soluble and one vitamin that is fat soluble. Explain the differences in solubility in terms of their structures and intermolecular forces. ................................................................................................................................................ ................................................................................................................................................ ................................................................................................................................................ ................................................................................................................................................ ................................................................................................................................................ ................................................................................................................................................ ................................................................................................................................................ ................................................................................................................................................ (Total 4 marks) 6. Vitamins C and D are vital in a balanced diet. State one major function of each of these vitamins and state a disease that results from the deficiency of each one. vitamin C function ................................................................................................................................................ ................................................................................................................................................ disease ................................................................................................................................................ ................................................................................................................................................ vitamin D function ................................................................................................................................................ ................................................................................................................................................ disease ................................................................................................................................................ ................................................................................................................................................ (Total 4 marks) 5