Module Description Template
advertisement
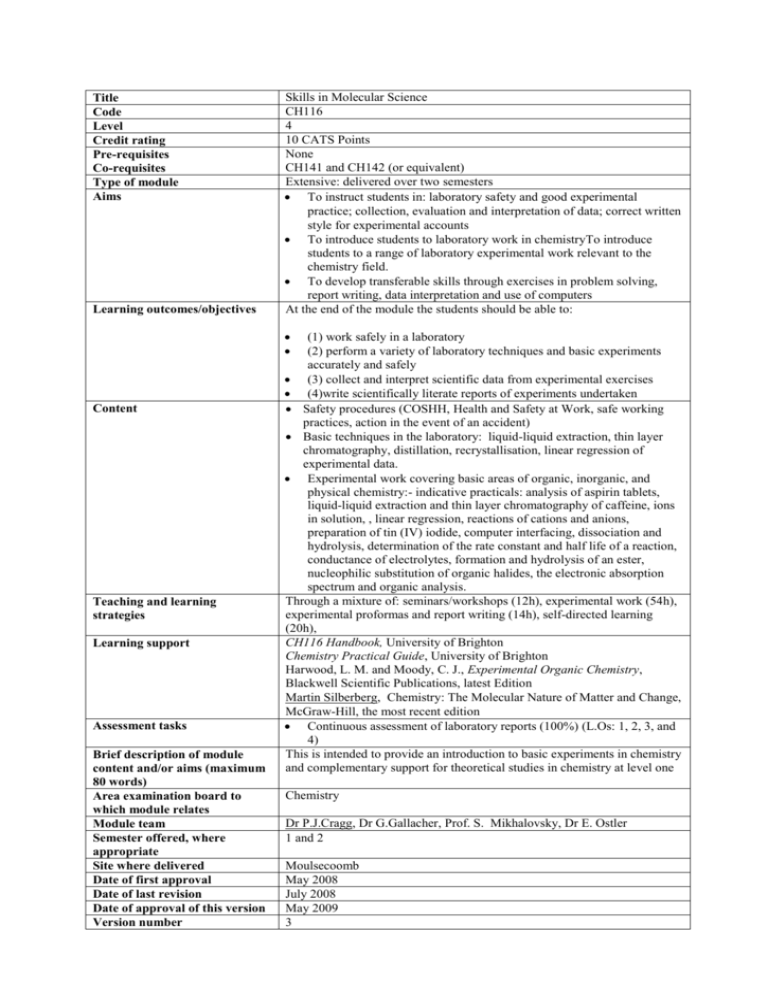
Title Code Level Credit rating Pre-requisites Co-requisites Type of module Aims Learning outcomes/objectives Skills in Molecular Science CH116 4 10 CATS Points None CH141 and CH142 (or equivalent) Extensive: delivered over two semesters To instruct students in: laboratory safety and good experimental practice; collection, evaluation and interpretation of data; correct written style for experimental accounts To introduce students to laboratory work in chemistryTo introduce students to a range of laboratory experimental work relevant to the chemistry field. To develop transferable skills through exercises in problem solving, report writing, data interpretation and use of computers At the end of the module the students should be able to: Content Teaching and learning strategies Learning support Assessment tasks Brief description of module content and/or aims (maximum 80 words) Area examination board to which module relates Module team Semester offered, where appropriate Site where delivered Date of first approval Date of last revision Date of approval of this version Version number (1) work safely in a laboratory (2) perform a variety of laboratory techniques and basic experiments accurately and safely (3) collect and interpret scientific data from experimental exercises (4)write scientifically literate reports of experiments undertaken Safety procedures (COSHH, Health and Safety at Work, safe working practices, action in the event of an accident) Basic techniques in the laboratory: liquid-liquid extraction, thin layer chromatography, distillation, recrystallisation, linear regression of experimental data. Experimental work covering basic areas of organic, inorganic, and physical chemistry:- indicative practicals: analysis of aspirin tablets, liquid-liquid extraction and thin layer chromatography of caffeine, ions in solution, , linear regression, reactions of cations and anions, preparation of tin (IV) iodide, computer interfacing, dissociation and hydrolysis, determination of the rate constant and half life of a reaction, conductance of electrolytes, formation and hydrolysis of an ester, nucleophilic substitution of organic halides, the electronic absorption spectrum and organic analysis. Through a mixture of: seminars/workshops (12h), experimental work (54h), experimental proformas and report writing (14h), self-directed learning (20h), CH116 Handbook, University of Brighton Chemistry Practical Guide, University of Brighton Harwood, L. M. and Moody, C. J., Experimental Organic Chemistry, Blackwell Scientific Publications, latest Edition Martin Silberberg, Chemistry: The Molecular Nature of Matter and Change, McGraw-Hill, the most recent edition Continuous assessment of laboratory reports (100%) (L.Os: 1, 2, 3, and 4) This is intended to provide an introduction to basic experiments in chemistry and complementary support for theoretical studies in chemistry at level one Chemistry Dr P.J.Cragg, Dr G.Gallacher, Prof. S. Mikhalovsky, Dr E. Ostler 1 and 2 Moulsecoomb May 2008 July 2008 May 2009 3 Replacement for previous module Field for which module is acceptable and status in that field Course(s) for which module is acceptable and status in that course School home External examiner CH110 Chemistry; compulsory Pharmaceutical and Chemical Science: compulsory Analytical Chemistry with Business: compulsory School of Pharmacy and Biomolecular Sciences Dr I Pulford







