Name
advertisement

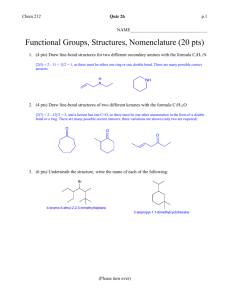
Houseknecht Chem 201 Fall 2012 Name_____________________________________ I _______________________________________ (sign name) affirm that my work upholds the highest standards of honesty and academic integrity at Wittenberg, and that I have neither given nor received any unauthorized assistance. There are 6 pages (including this one) and 10 questions on this exam. Read each question carefully before beginning. Show work for partial credit. A periodic table is attached to the end of this exam. You may remove it and use it for scrap paper. You may also use the lower portion of this cover page as scrap paper. If you would like any more scrap paper please ask. Good luck. Houseknecht Chem 201 Fall 2012 Exam 2 – October 9, 2012 1. (20 pts) Provide the IUPAC name or structure of the following compounds. Indicate stereochemistry if relevant. Structure Name Houseknecht Chem 201 Fall 2012 2. (6 pts) Circle the highest priority groups on each of the alkene carbons below and indicate whether each alkene is E or Z. 3. (4 pts) Calculate the degrees of unsaturation present in a molecule with the molecular formula C5H9NO2 and draw one possible structure. 4. (6 pts) Provide curved arrows to show how electrons move in the following reactions: 5. (4 pts) Identify one electrophilic and one nucleophilic site in testosterone. Houseknecht Chem 201 Fall 2012 Houseknecht Chem 201 Fall 2012 Energy 6. (6 pts) Label a transition state (TS) and an intermediate (I) for the reaction represented by a dashed line below. Reaction Progress a. Indicate which product (solid or dashed) would be favored if the reaction was reversible and explain your rationale. b. Indicate which product (solid or dashed) would be favored if the reaction was irreversible and explain your rationale. 7. (6 pts) Provide at least six unique statements explaining why only the products shown below are formed and why the major product is favored. Chemical structures will be important. Houseknecht Chem 201 Fall 2012 8. (10 pts) Reaction of the alkene below with HBr yields three products. Indicate how the first two products are formed using curved arrows to indicate electron flow for the cleavage and formation of all bonds. Also, explain why the minor product is not preferred. 9. (8 pts) Indicate how trans-1,2-dichlorocyclohexane is formed in the reaction below using curved arrows to indicate electron flow for the cleavage and formation of all bonds. Also, explain why the cis-1,2-dichlorocyclohexane is not observed. Houseknecht Chem 201 Fall 2012 10. (30 pts) Provide the major and minor organic products of the following reactions. If a reaction would not proceed, write “no reaction”. Indicate stereochemistry where important. Extra Credit (5 pts) A hydrocarbon of unknown structure has the formula C8H10. On catalytic hydrogenation over Lindlar’s catalyst, 1 eq. of H2 is absorbed. On hydrogenation over Pd/C, 3 eq of H2 are absorbed. How many degrees of unsaturation are present in the unknown? How many triple bonds are present? How many double bonds are present? How many rings are present? Draw a structure that fits this data.