histologyofurinarysystem-2009
advertisement

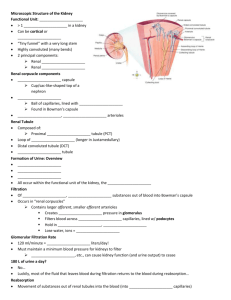
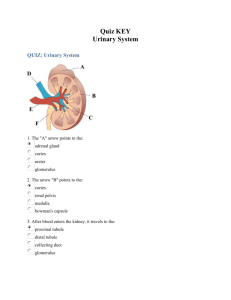
THE RENAL AND BODY FLUID A VIEW FROM HISTOLOGICAL ASPECT Ahmad Aulia Jusuf, MD, Ph.D Department of Histology Faculty of Medicine University of Indonesia 2009 INTRODUCTION The urinary system (Figure-1.1) consists of two kidneys, two ureters, a bladder and a urethra. Urine is produced in the kidneys and flows down the ureters to the bladder where it is stored until voided into the urethra.The kidney and ureters are located in the retroperitoneum while the urinary bladder is in the anterior part of the pelvis. The main function of the urinary system is the maintenance of water and electrolyte homeostasis which requires that any input into the system is balanced by equivalent output. The urinary system Image removed due to provides the mechanism by which excess water and copyright restriction electrolyte are eliminated from the body. The second major function of the urinary system is the excretion of Figure-1 The Urinary System many toxic metabolic waste products. The end product of these processes is urine. These actions are performed by the two kidneys. Urine is delivered from kidneys into the two ureters, from where it passes to the storage organ, the urinary bladder. During voiding the urin is deliver from the urinary bladder to the outside of the body via the urethra. In the female the urethra is solely a urinary duct but in the male, it also serves as the pathway for ejaculation of the semen. Additionally the kidneys have an endocrine function in that they produce renin which is important in the regulation of blood pressure and erythropoietin which acts on the bone marrow to stimulate production of erythrocytes. They are also involved in converting a circulating precursor of vitamin D to the active vitamin that plays important role in controlling the level of calcium in the body fluids. Blood is supplied to each kidney by renal arteries which arise from the aorta. One or more renal veins drain each kidney to the inferior vena cava. The total blood volume of the body is circulated through the kidneys about 300 times each day. Ahmad Aulia Jusuf/ Urinary System /Dept. of Histology, FMUI 2 KIDNEY Kidney is a large, reddish, bean-shaped organ lying in the upper retroperitoneal area. Because of the position of the liver, the right kidney is approximately 1 to 2 cm lower than the left. The size of kidney is about 11 cm long, 4 to 5 cm wide and 2 to 3 cm thick. It is wrapped by the perirenal fat. Branches of the renal artery and vein, lymph vessels, and ureter pierce the kidney in the fissure located the median border of each kidney, called as hilus. The ureter is expanded at this region forming the renal pelvis. Hemisection view of the kidney (Figure-2) shows that the archetypal kidney of the human consists of cortex and medulla. Cortical region appears dark-brown and granular, whereas the Image removed due to copyright medulla contains 6 to 12 discrete, restriction pyramid-shape, pale, striated regions, the renal pyramids. The base of each renal Fig-2 Hemisection view of the kidney pyramid is oriented toward the cortex, constituting the corticomedullary border, whereas its apex (known as renal papilla) is pointed toward the hilus. The apex is perforated by 20 or so openings of ducts of Bellini. This sieve-like region is known as the area cribosa. The apex is surrounded by a cup like shape known as minor calyx. Two or three minor calyces form a major calyx. Three or four major calyces empty into the renal pelvis. The lateral boundaries of each pyramid are defined darker inward extensions from the cortex, known as the cortical columns of Bertini. Cortex of the kidney is composed of 3 structure the renal corpuscle, proximal and istal convoluted tubules and longitudinal striations known as medullary rays (also called as processus Ferreini), which are cortical continuations of material located in the renal pyramids. One renal pyramid and its bounding renal columns constitute a renal lobe. Hence the kidney is a multilobar organ. Each medullary ray and its surrounding convoluted tubule is consider as a kidney lobule, which continues into the medulla as a cone-shape structure. URINIFEROUS TUBULES The functional unit of kidney is the uriniferous tubule (Figure-3), a highly convoluted structure that modifies the fluid passing through it to form urine as its final output. This Ahmad Aulia Jusuf/ Urinary System /Dept. of Histology, FMUI 3 functional unit consists of two parts, the nephron and collecting tubule (collecting system). There are approximately 1.3 million nephrons in each kidney. Several nephrons are drained by a single collecting tubules. Multiple collecting tubules join in the deeper aspect of the medulla to form the larger ducts. The largest of these ducts, the ducts of Bellini perforate the renal papilla at the area cribrosa. Image removed due to copyright restriction Figure-3 The uriniferous tubule NEPHRON In the human kidney, there are two type of nephrons: 1. The shorter cortical nephrons, which have both the renal corpuscle and the very short tubular parts in the cortex of the kidney. 2. The longer juxtamedullary nephrons which have the renal corpuscle in the cortex of kidney and the tubular parts in the medulla. The nephron is consisted of 2 components; the renal corpuscle (Malphigian corpuscle) and the renal tubule. The renal corpuscle or Malphigian corpuscle (Figure-4) is responsible for filtration of plasma. The renal corpuscle is composed of a tuft of capillaries, the glomerulus which is invaginated into Bowman’s capsule, the dilated pouch like, proximal end of the nephron. Renal tubule consists of a long tubular portion consisting of several regions, including a proximal convoluted tubule, a thick descending limb of Henle, a thin descending limb of the loop of Henle, a thin ascending limb of Henle, an ascending thick limb of Henle and a distal convoluted tubule that empties into collecting tubule. Image removed due to Image removed due to copyright restriction copyright restriction Figure-4 Malphigian corpuscle (Left side is the light microscopic appearance of Malphigian corpuscle, Right side is the schematic picture of Malphigian corpuscle GLOMERULUS Ahmad Aulia Jusuf/ Urinary System /Dept. of Histology, FMUI 4 The glomerulus (Figure 3,4 and 5) is a globular network of anastomosing capillaries that arise from the branches of the afferent glomerular arteriole which invaginates Bowman’s capsule. The region in where the blood vessels supplying and draining the glomerulus, enter and exit Bowman,s capsule is known as the vascular pole. Within the capsule, the glomerulus is invested by a layer of epithelial cells called podocytes, which constitute the visceral layer of Bowman’s capsule. The visceral layer is reflected around the vascular stalk of the glomerulus to become continuous with the parietal layer which constitute Bowman’s capsule. The capillaries constituting the glomerulus (Figure-5) are similar to the fenested type of capillaries. The endothelium of the glomerulus capillaries is thin and contains numerous number of large round fenestrated with the diameter of 70-100 nm. Unlike other fenestrated capillaries, the pores of the glomerulus capillaries do not have a pore diaphragma as infenestrated capillaries elsewhere in the body. Another unusual feature is that the luminar surface of the endothelium is negatively charged due to the present of a surface layer of a glycoprotein called as podocalxin. The thick portion of the endothelial cell bodies, containing the nucleus, is usually located on the side of the capillaries away from the capsule space. Image removed due to copyright restriction Figure-5 Fenestrated type capillar in the glomerulus, podocyte and filtration barrier The space between the glomerular capillaries (Figure-6) are occupied by the mesangium, a connective tissue consisting of mesangial cells in an extracellular matrix that is relatively free fibrous elements other than fibronectin. The specialized property of mesangial cells are 1. considered as to be a specialized type of pericyte providing structural support for the capillary loop. Unlike other pericyte they are phagocytic and it is speculated that they may be participate in the continuous turn over of the basal lamina by removing its outer portion containing residues of filtration, while the lamina basal is renew on its inner surface by the endothelial cells. 2. they are contractile and respond to angiotensin II and other vasoconstrictor that are known to reduce the area of the intraglomerular filtration barrier by reducing the blood flow through some of the capillary loops. Ahmad Aulia Jusuf/ Urinary System /Dept. of Histology, FMUI 5 3. have a specific receptor for the atriopeptides hormone which is secreted by certains cells of the myocardium. This hormone functions in regulating the vasodilation of some capillary loop near the vascular pole of the glomerulus. Image removed due to copyright Image removed due to copyright restriction restriction Figure-6 Glomerular capiller, podocyte and mesangial intraglomerular cells THE BOWMAN’S CAPSULE The Bowman’s capsule (Figure-7) consists of an inner visceral layer made up of highly branched cells called podocytes (Figure- 5 and 7) that completely invest the glomerular capillary. The podocytes have a long cytoplasmic extensions called primary processes which embrace the capillaries, giving rise to short secondary foot processes (pedicles) which interdigitate with those of other primary processes. The secondary foot processes are directly applied to the lamina rara externa and bound to it by fine filaments. The gaps between adjacent secondary foot processes, known as filtration slits are of uniform width (20-40 nm) and are bridge by a delicate electron-dense diaphragm 4 nm thick, called as filtration slit diaphragma (Figure- 5 and 8). The slit diaphragma has circular pores bridgeed by spoke-like configurations that radiate from a central density. These spoke are separated from one another by 3 to 5 nm spaces. The pores between them are believed to be small enough to prevent passage of albumin and larger molecules from the blood into the glomerular filtrate. Image removed due to The plasma membrane of the foot-processes has a copyright restriction prominent glycocalyx that is negatively charged and stains intensely with ruthenium red. This coat has Figure-7. Bowman capsule now been isolated and identified as a 140 kD sialoglycoprotein that is called podocalyxin. In the living state, the filamentous molecules forming the glycocalyx on adjacent foot processes, probably largely fill the filtration slits. So a layer of negatively Ahmad Aulia Jusuf/ Urinary System /Dept. of Histology, FMUI 6 charge podocalyxin covers the urinary surface of the podocytes, including the filtration slit. The high negatively of the surface coat could be a significant component of the filtration barrier. In electrone micrographs of thin sections, the nucleus of the podocytes is often infolded and irregular in outline. Cytoplasm of podocyte contains a small Image removed due to copyright Golgi complex, a moderate number of of restriction cisternal profiles of rough endoplasmic reticulum, and abundant free polyribosomes. Intermediate filament and microtubules are Figure-8 The Electrone microscopy picture of filtration slit plentiful, both in the cell body and in its primary processes. Actin filaments and heavy meromyosin have been localized cytochemically in the bases of the foot-processes. Since the podocyte cytoplasm contains actin filaments it seems that podocytes have contractile and phagocytic functions. The outer layer is called the parietal layer (Figure-4, 6 and 7)consists of simple squamous epithelium that is continuous at the urinary pole of the renal corpuscle with the proximal convoluted tubule. The space between the visceral and parietal layers of Bowman’s capsule is called the urinary space of Bowman. The primitive urinary filtrate passes into the urinary space and then into the proximal convoluted tubule. The region of continuation between the renal corpuscle and the proximal tubule, which drains Bowman’s space called as the urinary or tubular pole. GLOMERULAR BASEMENT MEMBRANE The glomerular basement membrane (240-340 nm) (Figure-5 and 8) also called as basal lamina is much thicker than other basement membranes and appears to be elaborated by both capillary endothelial cells and podocytes. As with basement membranes elsewhere, it consists of a feltwork of type IV collagen, structural glycoproteins (fibronectin and laminin) and proteoglycans rich in heparin sulphate. By electron microscopy, the glomerular basement membrane consists of three layers Ahmad Aulia Jusuf/ Urinary System /Dept. of Histology, FMUI 7 1. Lamina rara externa, located between lamina densa and the visceral layer of Bowman’s capsule.This layer contain laminin, fibronectin and a polyanionic proteoglycan and rich in heparin sulfate. 2. Lamina densa, the middle dense layer with the thickness of 300 nm and consists of type IV collagen. 3. Lamina rara interna, located between the lamina densa and the endothelial cells of the capillaries. This layer contains the structure similar to lamina rara externa. Fibronectin and laminin help pedicels and endothelial cells to maintain their attachment to the lamina densa. HISTOPHYSIOLOGIC OF GLOMERULAR FILTRATION The continuous filtration of the blood plasma in renal glomeruli is a process that is essential for the elimination of nitrogenous wastes and control of the extracellular fluid composition and of blood volume. The structural components of the filter barrier (Figure-5 and 8) are: 1. The fenestrated endothelium 2. The basal lamina 3. The filtration slits between the foot processes of the podocytes. Which of these components is the primary filter serving to retain plasma proteins in the circulation is still debated, but it is the prevailing view that the endothelial pores are only a corse sieve holding back the formed elements of the blood and that the basal lamina is the main filter. All layers contribute to the selective filtration process. Clinical evidence demonstrates that free haemoglobin and smaller molecules pass freely through the glomerular filter, whereas albumin and larger molecules are retained. For macromolecules three factors determine permeability, namely electrical charge, size and configuration. Negatively charged (cationic) molecules are blocked by the negatively charge endothelial cell coat and lamina rarae of the basement membrane, while the meshwork of lamina densa of the basement membrane discriminates on the basis of molecular size and configuration. The filtration slit diaphragm restricts the passage of any large molecules but its main role is in controlling water flow which is also held back by the colloidal osmotic pressure of retained albumin and other large molecules. The phagocytic function of the podocyte is to remove any large molecules which become trapped in the outer layers of the filter. Molecules trapped on the endothelial side are phagocytosed by mesangial cells. Ahmad Aulia Jusuf/ Urinary System /Dept. of Histology, FMUI 8 THE DEVELOPMENT OF NEPHRON During embryonic development, renal tubule (Figure- 9) develops from the embryological metanephros , as blind-ended tubes consisting of a single layer of cuboidal epithelium. The ends of the tubules dilate and become invaginated by a tiny mass of mesodermal tissue which differentiates to form the glomerulus. The layer of invaginated epithelium flattens and differentiates into podocytes which become closely applied to the surface of the knot of glomerular capillaries. Most of the intervening tissue disappears so that the basement membrane of glomerular endothelial cells and podocytes effectively fuse, forming the glomerular basement membrane. A small amount of tissue remains to support the capillary loops and differentiates to form the mesangium, a membrane like material that fills the space between the capillary loops in each glomerular. This Mesangium contains the mesangial cells. Image removed due to copyright restriction Figure-9 CORTEX OF THE KIDNEY The cortex of kidney is covered by a capsule, perirenal connective and adipose tissues. The cortex of kidney consists of renal corpuscle and adjacent proximal and distal tubules together with interlobular arteries and veins, juxtaglomerular complex and the medullary rays (processus of Ferreini) which contain the loop of Henle and collecting tubule. PROXIMAL TUBULE Image removed due to copyright Image removed due to copyright restriction restriction Figure-10 Proximal (PCT) and distal (DCT) convulated tubules and macula densa (MD) Bowman’s space drains into the proximal tubule at the urinary pole. In this junctional region, sometimes called the neck of the proximal tubule (negligible in human), the simple squamous epithelium of parietal layer of Bowman’s capsule joins the simple cuboidal epithelium of tubule. The proximal tubule consists of a highly tortous region, the pars convoluta (proximal convoluted tubule) (Figure-3 and 10), located near the renal corpuscle and a straighter portion the Ahmad Aulia Jusuf/ Urinary System /Dept. of Histology, FMUI 9 pars recta , which descend in the medullary rays within the cortex and then in medulla to become continuous with the Henle’s loop at outer parts of medulla. The proximal convoluted tubule (Figure-3 and 10) is a coil tube, widest segmen of the nefron with the length of 14 nm and stains acidophilic. The proximal convoluted tubule is cut in the various shapes. The wall of this tubule composed of simple cuboidal cells with the unclear boundaries. The nucleus of the cell is round in shape, blue, and located slightly far from the others. The cytoplasm of the cell is acidophilic in color due to its granule. The surface of cell toward to the lumen contains a well-developed brush border. The proximal convoluted tubule is responsible for the reabsoption of approximately 80% of water fluid including all the proteins, amino acids, glucose, and most ions and electrolytes (sodium, chloride, calcium, phosphate) from the tubular filtrate. There is an active transport of Na+ ions through the base of the cells into the interstitium; these ions then enter the peritubular capillaries. The many mitochondria operate an Mg2+-dependent Na+, K+ activated ATPase pump located in the basal part of the cell membrane. Chloride ions passively follow the actively transported Na+ ions. The accumulation of ions outside the base of the cell causes water to move passively out of the tubule lumen; this water is called obligatory water. Because the proximal convoluted tubule reabsorbs sodium and chloride at the same rate as water, the filtrate that enters the loop of Henle has the same osmotic pressure as that which entered the proximal convoluted tubule from the urinary space. Additionally all of the glucose, amino acids, and protein in the glomerular ultrafiltrate are resorbed by cells of the proximal tubule. DISTAL TUBULE The distal tubule is subdivided into the pars recta which as as the continuation of the ascending thin limb of Henle’s loop is also known as the ascending thick limb of Henle’s loop and the pars convoluted (distal convoluted tubule) (Figure-3 and 10). Interpose between the ascending thick limb and the distal convoluted tubule is a modified region of the distal tubule known as the macula densa (Figure-3 and 10). The ascending thick limb of Henle’s loop is 9 to 10 nm in length and 30-40 m in diameter. It joins the ascending thin limb at outer part of the medulla and ascends straight up through the medulla to reach the cortex. The thick ascending limb is not permeable to water or urea. Its cell have chloride (and perhaps sodium) pumps that function in the active transport of chloride (and sodium) from the lumen of the tubule. Thus as the filtrate reaches the cortex of the kidney within the lumen of the distal tubule, its salt concentration is low and its urea concentration remains high. Ahmad Aulia Jusuf/ Urinary System /Dept. of Histology, FMUI 10 As the ascending thick limb of the Henle loop pass near its own renal corpuscle, it lies between the afferent and efferent glomerular arterioles. This region of the distal tubule is called the macula densa (Figure-10). Because the cells of the macula densa are tall and narrow, the nuclei of these cells appear to be much closer together than those of the remainder of the distal tubule. The wall of the distal convoluted tubule (Figure-10) composed of simple cuboidal cells with the clearer boundaries compared to the proximal tubule. The nuclei of the cells are round in the shape with the blue color. The distant of the nuclei between the cells is closer compared to the proximal convoluted tubule. The cytoplasm is blue in color due to the presence of basophilic granule. The surface of the cells toward to the lumen does not contain the brush border. The lumen of the distal tubule is larger and is more clearly defined lumen, more nuclei per-cross section The distal convoluted tubule is mainly involved in reabsorption of sodium ions from the tubular fluid. The process is directly coupled to the secretion of hydrogen and potassium ions into the tubular fluid, one hydrogen ion or one potassium ion being secreted for every sodium reabsorbed; in this way the distal convoluted tubule plays an important role in acid-base balance. This process is controlled by the hormone aldosterone secreted by the adrenal cortex. A certain amount of potassium is also reabsorbed in the distal convoluted tubule. JUXTAGLOMERULAR COMPLEX The Juxtaglomerular complex (Figure-11) is made up of tubular and vascular elements of the nephron that have interactive functions influencing systemic blood pressure and the rate of glomerular filtration. In histological sections the distal convoluted tubule of nephron is located at the vascular pole of the glomerules, between the afferent and efferent arteriole, the epithelial cells are slender and more crowded than elsewhere, called as macula densa. The cells of the macula densa are tall, narrow, pale cells with centrally placed nuclei. Because of the narrowness of these cells, the densely staining nuclei are near to each other; collectively, viewed with the light microscope, they appear as a dense spot. The basement membrane between the macula and underlying cells is extremely thin and discontinuous. The blunt cell processes are reported to extend through it toward the juxtaglomerular cells in the afferent arteriole. These relationships strongly suggest that the macula densa has a sensory function that influences the activity of the juxtaglomerular cells. The cells of the macula densa are thought to be sensitive to the concentration of sodium ions in the fluid within the distal convoluted tubule. Decreased in systemic blood pressure results Ahmad Aulia Jusuf/ Urinary System /Dept. of Histology, FMUI 11 in decreased production of glomerular filtrate and hence decreased concentration of sodium ions in the distal tubular fluid. Juxtaglomerular cells are modified smooth muscle cells in the tunica media of the afferent arteriole close to the renal corpuscle. These cells form a cluster around it just before it enters the glomerulus. Juxtaglomerular cell cytoplasm contains the granules of renin and erythropoietin. Erythropoietin is a hormone that stimulates maturation of red blood cells in the bone marrow in response to reduced oxygen tension. Renin, an aspartly peptidase enzyme, plays an important role in water conservation and regulation of blood pressure. Extraglomerular mesangial cells (Goormaghtigh or Lacis or Polkissen cells) form a conical mass, the apex of which continuous with the mesangium of the glomerulus, laterally it is bounded by the afferent and efferent arterioles and its base abuts the macula densa. The lacis cells are flat and elongated with extensive fine cytoplasmic processes extending from their ends and surrounded by a network of mesangial material. Despite the central location in the juxtaglomerular, the function of the extraglomerular mesangial cells is not yet clear. The current theory is that these cells participate in the tubuloglomerular feedback mechanism by which changes in Na+ concentration at the macula densa give rise to signals which directly control glomerular blood flow. The extraglomerular mesangial cells are thought to be responsible for transmission of a signal arising in the macula densa to the intraglomerular mesangial cells which then contract or relax to make the capillary loops narrower or wider. Image removed due to copyright Image removed due to copyright restriction restriction Image removed due to copyright restriction Figure-11 Juxtaglomerular apparatus (macula densa, lacis/Polkissen and juxtaglomerular cells) THE ROLE OF JUXTAGLOMERULAR APPARATUS IN THE CONTROL OF BLOOD PRESSURE The juxtaglomerular apparatus (Figure-11) is believed to act as both a baroreceptor and a chemoreceptor, controlling systemic blood pressure by the secretion of renin by the Ahmad Aulia Jusuf/ Urinary System /Dept. of Histology, FMUI 12 juxtaglomerular cells. The juxtaglomerular cells are suitably placed to monitor systemic blood pressure, with a fall in blood pressure resulting in renin secretion. Reduction in blood pressure results in reduced glomerular filtration (Figure-12) and consequently a lower concentration of sodium ions in the distal convoluted tubule. Acting as chemoreceptors the cells of the macula densa in some way then promotes renin secretion. Renin diffuses into the bloodstream catalyzing the conversion of angiotensinogen, an alpha2 globulin synthesized by the liver into the decapeptide angiotensin I. In the lungs, angiotensin converting enzyme produced by endothelial cells cleaves two amino acids from angiotensin I to form angiotensin II which is a potent vasoconstrictor. Angiotensin II raises blood pressure in three ways: constriction of peripheral blood vessels, release of aldosterone from the adrenal cortex and via a direct effect on the renal tubules where it promotes the reabsoption of sodium ions from the distal convoluted Image removed due to copyright restriction tubule, thus expanding the plasma volume Figure-12 The regulation of blood pressure by juxtaglomerular apparatus and increasing blood pressure. The tubuloglomerular feedback mechanism is also thought to operate at a local level to control glomerular blood flow and therefore indirectly systemic blood pressure. THE MEDULLA OF KIDNEY The medulla of kidney is composed of a number of renal pyramids. Each pyramid lies with its base adjacent to cortex and its apex directed inward. The medulla contains the loops of Henle, collecting tubule of koligens, collecting duct or papillary duct of Bellini, renal papilla, cortical collumn of Bertini, calyx minor and interlobaris arteries/veins. Ahmad Aulia Jusuf/ Urinary System /Dept. of Histology, FMUI 13 LOOP OF HENLE The loop of Henle (Figure-3 and 13) is made up of four parts: 1. the thick descending limb (Pars recta of the proximal tubule) 2. The thin descending limb 3. The thin ascending limb 4. The thick ascending limb (Pars recta of the distal tubule) The pars recta is the second, straight part of the proximal tubule which extends down into the outer medulla. There is an abrupt transition to the thin descending Image removed due to copyright limb which loops down into the medulla restriction for a variable distance. The thin limb of juxtamedullary nephrons extend down to Figure-13 Medulla of Kidney (CD= collecting duct of Bellini; T= thin limb of Henle loop; A= thick limb of Henle loop, CT = Collecting tubule of Koligens; V= vein) the inner medulla before turning back on themselves , while the thin limbs of cortical nephron only extend a short way into the medulla. After the hairpin bend the tubule becomes the thin ascending limb for a short distance before abruptly changing into the thick ascending limb. Thus the thin descending limb is longer than the thin ascending limb. The pars recta of the proximal tubule has the appearance similar to the proximal convolute tubule but its diameter is smaller than that of proximal convoluted tubule. The thin limb (descending and ascending) has the appearance similar to the blood capillary with a simple squamous epithelium but the epithelium slightly thicker than that of capillary. The lumen may be differentiated from the vasa recta by the absence of erythrocyte and their regular rounded in transverse section. Thick ascending limb of Henle has the appearance similar to distal convoluted tubule.This tubule is lined by low cuboidal epithelium and are also round in cross sections. Neither thick nor thin limb of the loop of henle have a brush border. The function of the loop of Henle is to produce an increasing osmotic gradient from the cortex to the tip of renal papilla by the counter-current multiplier mechanism. The parts of the loop of Henle with a thick (cuboidal) epithelium participate in active transport of various ions and molecules out of the lumen and into the interstitium. On the other hand the thin limb are lined by a flattened squamous epithelium which has no capacity for active Ahmad Aulia Jusuf/ Urinary System /Dept. of Histology, FMUI 14 transport. The thin descending limb allows free diffusion of H2O but is fairly impermeable to NaCl, while the thin ascending limb is permeable to to NaCl but not to H2O. The vasa recta take up water from the medullary interstitium and return it to the general circulation. As the urine flows into the thick ascending limb, active transport of NaCl again occurs and this correlates with the appearance of the epithelium. The thick ascending limb is also impermeable to water which may be related to its thick glycocalyx composed of the glycoprotein. COLLECTING TUBULE AND DUCTS The collecting tubule of koligen (Figure-3 and 13) joins the distal convoluted to collecting duct. Several collecting tubules merge to form each collecting duct. The collecting tubule descend in the medullary rays toward the renal medulla where they progressively merge to form the large collecting ducts of Bellini (Figure-3 and 13) which drain urine from the tip of renal papilla into the pelvic-calyceal system. The collecting tubule of koligens (Figure-13) has the appearance similar to distal convoluted tubule but their epithelium are lightly stained cuboidal cells with visible cell membranes. The collecting tubules coalesce as they pass through the medulla to form large papillary ducts. The cuboidal epithelium of collecting tubule becomes increasingly tall distally until it merges with the columnar epithelium of the collecting duct. The simple columnar epithelium of the collecting duct or papillary ducts (Figure-13) consists of two cell types, the principle cells and intercalated cells. Principal cells have pale cytoplasm with scanty organelles with luminar short microvilli and a single cilium. There are prominent basal infoldings of the basolateral plasma membrane but no lateral Principal cells actively reabsorb Na+ and secrete K+ as well as reabsorbing water. Intercalated cells have darker interdigitations. cytoplasm due to the content of multiple mitochondria, polyribosome, and membrane bound vesicles. The intercalated cells function to secrete H+ and reabsorb bicarbonate and are thus important in acid-base homeostasis. The collecting tubule and papillary ducts concentrate urine by passive reabsorption of water into the medullary interstitium following the osmotic gradient created by the counter current multiplier system of the loop of Henle. The vasa recta return this water to the general circulation. The amount of water reabsorbed is controlled by antidiuretic hormone (ADH, vasopressin) secreted by posterior pituitary in response to dehydration. ADH acts by increasing the permeability to water of the collecting tubule and duct resulting in retention of water by the body and the production of hypertonic urine. Conversely, ADH secretion is inhibited by water overload and an increasing volume of hypotonic urine is thus produced. The collecting tubule and ducts are also the site of H+ secretion and therefore important in the maintenance of acid-base balance. Ahmad Aulia Jusuf/ Urinary System /Dept. of Histology, FMUI 15 The interstitium of the inner medulla in some species including human, contains unusual cells called lipid laden interstitial cells arranged at right angles to the tubules and vasa recta bound tightly to one another. The function of these cells is not yet clear but they may be involved in the production of prostaglandins and/or hormones which regulate blood pressure. CALYCES The renal papilla of each renal pyramid fits into a minor calyx, a funnel-shaped chamber that accepts the urine leaving the ducts of Bellini at the area of cribrosa. The portion of Image removed due to copyright restriction the apex of the pyramid that projects into the minor calyx is covered by transitional epithelium, which acts as a barrier, separating Figure-14 Papilla renalis and minor calyc the urine from the underlying connective tissue lamina propria. Deep to the lamina propria is a thin muscular coat, composed entirely of smooth muscle. This muscular layer propels the urine into a major calyx, one of three or four larger funnel-shaped chambers, each of which collects urine from two to four minor calyces. The major calyces are similar in structure to the minor calyces as well as to the expanded proximal region of the ureters the renal pelvis. The walls of the excretory passages thicken from the minor calyces to the urinary bladder. RENAL INTERSTITIUM The kidney is invested by a dense, irregular collagenous type of connective tissue, with some elastic fibers intersperse among the bundle of collagen. This capsul is not attached firmly to the underlying cortex. As the blood vessel enters the hillum, they travel in a thin connective tissue cover, some of which is derived from the capsule. The cortical region has only delicate connective tissue elements, mostly associated with the basement membranes investing the uriniferous tubules and their vascular supply. The two cellular components of the cortical connective tissue are fibroblass and cells that are probably macrophages. The medullary interstitial connective tissue component is more extensive than that found in the cortex. Embedded in this connective tissue are the various components of the uriniferous tubules as well as the extensive vascular network located in the medulla. The cell population of Ahmad Aulia Jusuf/ Urinary System /Dept. of Histology, FMUI 16 this connective tissue consists of three cell types: fibroblasts, macrophages, and interstitial cells. Interstitial cells appear to be placed like rungs of a ladder, one top of the other and are most numerous between straight collecting ducts and between the ducts of Bellini. Interstitial cells have elongated nuclei and numerous lipid droplets. It is believed that these cells synthesize medullipin I, a substance that is converted in the liver to medullipin II, a potent vasodilator that lowers blood pressure. RENAL VASCULATURE The kidney receives an extremely extensive blood supply via the large renal artery, a direct branch of the abdominal aorta. Before entering the hilum of the kidney, the renal artery bifurcates (Figure-15) into an anterior and a posterior division, which Image removed due to copyright in turn subdivide to form a total of five restriction segmental arteries. The first subdivisions of the segmental arteries are called lobar arteries, one for each lobe of the kidney. Figure-15 Vascularization of kidney These in turn branch to form two or three interlobar arteries, which travel between the renal pyramids to the corticomedullary junction. At the corticomedullary junction the interlobar artery which run in an-like course parallel to the capsule of the kidney. Because these arteries describe a slight arc over the base of the renal pyramid, they are named arcuate arteries. The arcuate artery ascends into the cortex and gives rise to numerous cortical radial (interlobular) arteries which radiate toward the capsule, branching to form the afferent arterioles of the glomeruli and form the microcirculation of the renal medulla. The efferent’s arteriol which drains the glomerulus and leave the corpuscle form the vasa recta. The vasa recta descend into the medulla and make the microcirculation of the renal medulla. Some of the interlobular arteries ascend through the cortex to perforate the kidney capsule. Here they contribute to the formation of capsular plexus. The cortical and medullary capillaries drain via cortical radial (interlobular) veins to arcuate veins at the cortico-medullary junction and thence to the renal vein. Ahmad Aulia Jusuf/ Urinary System /Dept. of Histology, FMUI 17 LYMPHATIC SUPPLY OF THE KIDNEY The lymphatic supply of the kidney is not completely understood. It is believed that most lymphatic vessels follow the larger arteries. According to most investigators, the lymphatic supply of the kidney may be subdivided into superficial and deep aspects located in the subcapsular region and medulla, respectively. The two systems may or may not join each other near the hilum, where they form several large lymphatic trunks. Recently it has been demonstrated that there are lymph vessels in the cortex that follow the larger arteries, but they do drain their lymph into a plexus of lymph vessels at the hilum. RENAL INNERVATION Most nerve fiber that reached the kidney is unmyelinated, sympathetic fibers that form the renal plexus, traveling along the renal artery. The cell bodies of these fibers are probably located in the aortic and celiac plexuses. Sympathetic fibers are distributed by branches of the renal arterial tree, and these vessels are modulated by some of these fibers. Additional sympathetic fibers reach the epithelium of the renal tubules, the juxtaglomerular and interstitial cells, and the capsule of the kidney. Sensory fibers and parasympathetic fibers (probably from the vagus nerve) have also been described in the kidney. WATER CONSERVATION Water conservation is an important homeostatic mechanism, especially for land-dwelling animal, who have to cope with the constant problem of dessication. 80% of the water filtered into the nephron at Bowman’s capsule by glomerular filtration is reabsorbed in the proximal convoluted tubule by a mechanism that involves active transport of sodium chloride with water following from the filtrate by passive diffusion. Water conservation in the remainder of tubule (i.e. the loop of Henle’s countercurrent multiplier effect) (Figure-16) is a way of concentrating urine and conserving the last remaining water by means that can be regulated. The loop of Henle creates an environment for retention of water by producing a hypertonic urine and conserving body water. The loop of Henle creates a gradient of increasing hypertonicity or concentration that is lowest in the cortex and highest in the inner medulla. This hypertonic interstitium concentrates the urine as it flows through the collecting ducts that pass through the hyperosomotic kidney medulla. Sodium and chloride can enter the descending thick limb from the interstitium and are thereby available to pass once again into the thick ascending limb, to be repumped into the interstitium, thereby concentrating the interstitium and increasing the osmolarity of this region surrounding the tubules. Because of this hyperosmotic medulla, Ahmad Aulia Jusuf/ Urinary System /Dept. of Histology, FMUI 18 water is reabsorbed into the collecting tubules as they pass through the medulla. Because of the osmotic pressure, the water from the tubule lumen passes into the capillaries in the medullary interstitium and is conserved. Image removed due to copyright restriction The thin ascending limb, the thick ascending limb, and the initial part of the distal convoluted tubule are impermeable to water. The thick ascending limb actively transports chloride ions to the interstitium; there is also a passive reabsorption of sodium ions into the interstitium. This causes the tubule fluid in this location to be diluted but concentrates the interstitium, making it more hyperosmotic. Water is reabsorbed down its osmotic gradient in the distal portion of the distal convoluted tubule, as well as in the collecting tubule and ducts. Water and some urea are reabsorbed from the collecting duct in the inner medulla region, and some of the urea reenters the loop of Henle. Urea recycling tends to cause the ure to accumulated in the medullary interstitium, where it plays a role in extracting water from the descending limb so as to concentrate sodium chloride in the descending limb. Since nephrons in different regions of the cortex have tubules in different parts of the medulla, they can produce different concentrations of urine. Cortical nephrons produce a more dilute urine, while juxtamedullary nephrons produce a more concentrated urine. Sympathetic nerve stimulation causes contraction of sphincters in the afferent arterioles by shunting blood to predominantly juxtamedullary or cortical nephrons. The major transport events in the uriniferous tubule are summarized as below. ACID-BASE BALANCE Kidneys play an important role in maintaining the acid-base balance of the blood. The blood is maintained at a pH of approximately 7,4 by a buffering system that involves carbonic acid (H2CO3) and bicatbonate ions (HCO3-). If the blood become more acid (less alkaline), carbonic acid dissociates to hydrogen and bicarbonate ions. acidic H2CO3 ===== H+ + HCO3basic Ahmad Aulia Jusuf/ Urinary System /Dept. of Histology, FMUI 19 If the blood becomes less acid (more alkaline), the bicarbonate ions combine with the H+ ions to produce H2CO3. The secretion of H+ and NH3 and the reabsoption of Na+ and HCO3- are adjusted to maintain the pH within a normal range. If blood is acidic, H+ is excreted along with ammonia, while Na+ and HCO3- are reabsorbed. If the blood is basic, fewer H+ ions are excreted and fewer Na+ and HCO3- ions are reabsorbed. URETER Each ureter ( Figure- 17) is about 3 to 4 mm in diameter, is approximately 25 to 30 cm long and pierces the base of the urinary bladder. The ureters are hollow, cylindrical tubes, consisting of a mucosa, which lines the lumen, a muscular coat and a fibrous, connective tissue covering. The mucosa of the ureter presents several folds, which project into the lumen when the ureter is empty but that are absent when the ureter is distended. The transitional epithelial lining , three to five cell layers in thickness, overlies a layer of dense, irregular fibroelastic connective tissue which constitue the lamina propria. The muscular of ureter is composed of two predominantly inseparable layers of smooth muscle cells. The arrangement of the layers is opposite that found in the digestive tract, because the outer layer is arranged circularly and the inner layer is longitudinally disposed. This arrangement is true for the proximal two thirds of the ureter, but in lower third, near the urinary bladder, a third muscle layer, whose fibers are oriented longitudinally, is added onto the existing surface of the existing muscle coat. Hence the muscular fiber orientation in the lower one third of the ureter is outer longitudinal, middle circular, and inner longitudinal. The fibrous outer coat of the ureter is unremarkable and at its proximal and distal terminals, blends with the capsule of the kidney and the connective tissue of the bladder wall. Contrary to expectation, urine does not pass down the ureter because of gravitational force; instead, muscular contraction of the ureteric wall establishes peristaltic like waves that convey urine to the urinary bladder. As the ureter pierce the posterior aspect of the base of the bladder, a valve-like flap of mucosa hangs over each ureteric orifice, preventing regurgitation of urine from the bladder back into the ureters. Image removed due to copyright restriction URINARY BLADDER The urinary bladder (Figure- 18) is essentially an organ for storing urine until the pressure becomes sufficient to induce the urge for micturation, or voiding. Its mucosa also acts as an Ahmad Aulia Jusuf/ Urinary System /Dept. of Histology, FMUI 20 osmotic barrier between the urine and the lamina propria. The mucosa of the bladder is arranged in numerous folds which disappear when the bladder becomes distended with urine. During distension, the large, round, dome-shaped cells of the transitional epithelium become stretched and change their morphology to become flattened. The accommodation of cell shape is performed by a unique feature of the transitional epithelial cell plasmalemma, which is composed of a mosaic of specialized rigid, thickened Image removed due to copyright restriction Image removed due to copyright restriction Figure-18 Urinary bladder regions, plaques, interspersed by normal cell membrane, interplaque regions. When the bladder is empty, the plaque regions are folded into irregular, angular contours, which disappear when the cell becomes stretched. These rigid plaque regions, anchored to intracytoplasmic filaments, resemble gap junctions, but this similarity is only superficial. Plaques appear to be impermeable to water and salts; thus these cells acts as osmotic barriers between the urine and the underlying lamina propria. The superficial cells of the transitional epithelium are held together by desmosomes and possible, by tight junctions which also aid the establishment of the osmotic barrier by preventing the passage of fluid between the cells. The triangular region of the bladder, whose apices are the orifices of the two ureters and the urethra, is known as the trigone of the bladder. The mucosa of the trigone is always smooth and is never thrown into folds. The embryonic origin of the trigone differs from that of the remainder of the bladder. The lamina propria of the bladder may be subdivided into two layers: a more superficial, dense, irregular collagenous connective tissue and a deeper, looser layer of connective tissue composed of a mixture of collagen and elastic fibers. The lamina propria contains no gland except at the region surrounding the urethral orifices, where mucous glands may be found. Usually this gland extends only into the superficial layer of the lamina propria. They secrete a clear viscous fluid that apparently lubricates the urethral orifice. Ahmad Aulia Jusuf/ Urinary System /Dept. of Histology, FMUI 21 The muscular coat of the urinary bladder is composed of three interlaced layers of smooth muscle, which can be separated only in the region of the neck of the bladder. Here they are arranged as a thin, inner longitudinal layer, a thick middle circular layer forms the internal orifice of the urethra. The adventitia of the bladder is composed of a dense, irregular collagenous type of connective tissue, containing a generous amount of elastic fibers. Certain regions of the adventitisia are covered by a serosa, a peritoneal reflection onto the wall of the bladder, whereas other regions may be surrounded by fat. URETHRA The urinary bladder is drained by a single tubular structure, the urethra, which communicates with the outside, permitting elimination of urine from the body. As the urethra pierces the perineum, skeletal muscle fibers form the external sphincter muscle surrounding the urethra. This muscle permits voluntary control of micturation. The urethra of the male is longer than that of the female and has a dual function in that it acts as route for urine as well as for semen. Female urethra is about 4 to 5 cm in length and 5 to 6 mm in diameter. It extends from the urinary bladder to the external urethral orifice just above and anterior to the opening of the vagina. Normally the lumen is collapse, except during micturation. It is lined by a transtitional epithelium near the bladder and by a stratified squamous non keratinized epithelium along the remainder of its length. Interspersed in the epithelium are patches of pseudostartified columnar epithelium. The mucosa is arranged in elongated folds because of the organization of the fibroelastic lamina propria. Along the entire length of the urethra are numerous clear, mucussecreting glands of Littre. The male urethra (Figure-19) is 15 to 20 cm long, and its three regions are named according to the structure through which it passes. The segments are: the prostatic, membranous, and penile urethra. Image removed due to copyright restriction Figure-19 Male urethra Ahmad Aulia Jusuf/ Urinary System /Dept. of Histology, FMUI 22 The prostatic urethra 3 to 4 cm long lies entirely in the prostate gland. It is lined by a transitional epithelium and receives the openings of many tiny ducts of the prostate, the prostatic utricle (a rudimentary homologue of the uterus), and the paired ejaculatory ducts. The second segment of the male urethra is only 1 to 2 cm long and is known as the membranous urethra, because it passes through the perineal membrane (urogenital diaphragma). The membranous urethra is lined by stratified columnar epithelium, interspersed with patches of pseudostartified columnar epithelium. The final segment is the longest portion of urethra (15 cm in length). It passes through the length of the penis, terminating at the tip of the glands penis as the external urethral orifice. This segment is known as the spongiose urethra (penile urethra) because it is located in the corpus spongiose urethra (penile urethra) because it is located in the corpus spongiosum. The spongiose urethra is lined by stratified columnar epithelium interspersed with patches of pseudostratified columnar and stratified squamous non keratinized epithelia. The enlarged terminal portion of the urethra in the glands penis is known as the navicular fossa; it is lined by stratified squamous, nonkeratinized epithelium. The lamina propria of all three regions is composed of a loose fibroelastic connective tissue with a rich vascular supply. It houses numerous glands of Littre, whose mucous secretion lubricates the epithelial lining of the urethra. Ahmad Aulia Jusuf/ Urinary System /Dept. of Histology, FMUI